ISO Certifications for Pharmaceuticals Packing and Labelling Services, Requirements and Benefits
Pharmaceutical packaging and labeling are critical aspects of the drug supply chain. Ensuring compliance with international standards not only guarantees patient safety but also enhances product credibility in the global market. Regulatory bodies worldwide emphasize the need for standardized procedures to maintain consistency, prevent contamination & ensure clear labeling to minimize risks.
ISO provides globally recognized frameworks that help pharmaceutical companies establish strong quality management systems, improve efficiency and comply with regulatory requirements. For pharmaceutical packaging and labeling services, ISO certifications play a vital role in ensuring the integrity of drug products throughout their lifecycle.
Recently, the pharmaceutical industry is witnessing an increased focus on sustainable packaging solutions and digital labeling technologies. Companies are now integrating advanced security features in packaging to combat counterfeiting while maintaining compliance with ISO and regulatory standards.
Email Us – Reach out to our team at support@pacificcert.com for all certification inquiries.
Applicable ISO Standards for Pharmaceuticals Packing and Labelling Services
Several ISO standards are relevant to pharmaceutical packaging and labeling services. These standards ensure that companies follow best practices for quality and regulatory compliance. Below are the most applicable ISO standards:
ISO 9001:2015 – Quality Management System (QMS): ISO 9001:2015 sets the foundation for quality management in pharmaceutical packaging and labeling. It focuses on:
- Customer satisfaction
- Continuous improvement
- Risk management
- Process optimization
By implementing ISO 9001, companies can ensure consistent quality in packaging and labeling while meeting regulatory requirements.
ISO 15378:2017 – Primary Packaging Materials for Medicinal Products: This standard is specifically designed for pharmaceutical packaging manufacturers. It integrates ISO 9001 principles with Good Manufacturing Practices (GMP), ensuring that primary packaging materials meet quality and safety requirements. Key areas covered include:
- Risk management for contamination prevention
- Process validation and documentation
- Traceability of packaging materials
ISO 13485:2016 – Quality Management System for Medical Devices (Applicable to Drug-Device Combinations): For pharmaceuticals that incorporate medical devices (such as pre-filled syringes or drug-coated stents), ISO 13485 certification is crucial. It ensures compliance with regulatory requirements and focuses on:
- Product safety and risk management
- Documented processes for design and production
- Regulatory compliance for combination products
ISO 14001:2015 – Environmental Management System (EMS): Pharmaceutical companies are now adopting eco-friendly packaging solutions. ISO 14001 helps organizations minimize their environmental impact by:
- Reducing waste and emissions
- Implementing sustainable packaging materials
- Complying with environmental regulations
ISO 45001:2018 – Occupational Health and Safety Management System: This standard ensures a safe working environment for employees involved in packaging and labeling services. It focuses on:
- Hazard identification and risk assessment
- Employee health and safety policies
- Compliance with occupational safety regulations
ISO 27001:2022 – Information Security Management System (ISMS): With increasing digitization in pharmaceutical labeling, ensuring data security is critical. ISO 27001 helps companies protect sensitive data related to:
- Product labeling and serialization
- Electronic batch records
- Intellectual property and regulatory data
Click here to find out more applicable standards to your industry
At Pacific Certifications, we specialize in auditing and issuing ISO certifications for pharmaceutical packaging and labeling service providers. Our team of experienced auditors ensures that your organization meets the required standards through rigorous assessments and compliance verification.
As a certification body, we provide:
- Independent, third-party audits
- Certification issuance upon successful compliance
- Surveillance audits to maintain certification integrity
We do not offer consultancy, implementation or training services, ensuring complete impartiality in our certification process. Our goal is to help pharmaceutical companies to achieve internationally recognized compliance, enhancing their credibility in the market.
For certification inquiries, contact us at support@pacificcert.com.
Requirements of ISO Certifications for Pharmaceuticals Packing and Labelling Services
Each ISO standard has specific requirements that pharmaceutical packaging and labeling service providers must meet. Below is a breakdown of key requirements for each relevant standard:

ISO 9001:2015 – Quality Management System (QMS)
- Establish a documented quality management system (QMS).
- Define customer requirements and ensure consistent product quality.
- Implement risk-based thinking and continuous improvement.
- Maintain records of processes, quality checks, and corrective actions.
ISO 15378:2017 – Primary Packaging Materials for Medicinal Products
- Comply with Good Manufacturing Practices (GMP) for pharmaceutical packaging.
- Implement risk management for contamination and defects.
- Ensure material traceability from raw materials to finished packaging.
- Conduct regular quality audits and maintain process validation records.
ISO 13485:2016 – Medical Devices QMS (Applicable to Drug-Device Combinations)
- Establish documented procedures for medical packaging and labeling.
- Implement risk management for drug-device combination products.
- Ensure regulatory compliance and traceability of packaging materials.
- Conduct design and production validation processes.
ISO 14001:2015 – Environmental Management System (EMS)
- Identify and control environmental impacts of packaging materials.
- Implement waste reduction and sustainability initiatives.
- Comply with environmental laws and regulations.
- Monitor and improve resource efficiency (water, energy, raw materials).
ISO 45001:2018 – Occupational Health and Safety Management System (OHSMS)
- Identify workplace hazards in packaging and labeling operations.
- Implement safety policies to prevent workplace injuries.
- Conduct regular risk assessments and employee training.
- Ensure compliance with health and safety regulations.
ISO 27001:2022 – Information Security Management System (ISMS)
- Protect sensitive pharmaceutical labeling and serialization data.
- Implement cybersecurity measures for electronic labeling and batch records.
- Ensure access control and secure handling of confidential information.
- Conduct risk assessments for data breaches and cyber threats.
Meeting these requirements ensures compliance and efficiency in pharmaceutical packaging and labeling services.
Contact support@pacificcert.com to arrange an ISO certification audit tailored to your business needs.
Benefits of ISO Certifications for Pharmaceuticals Packing and Labelling Services
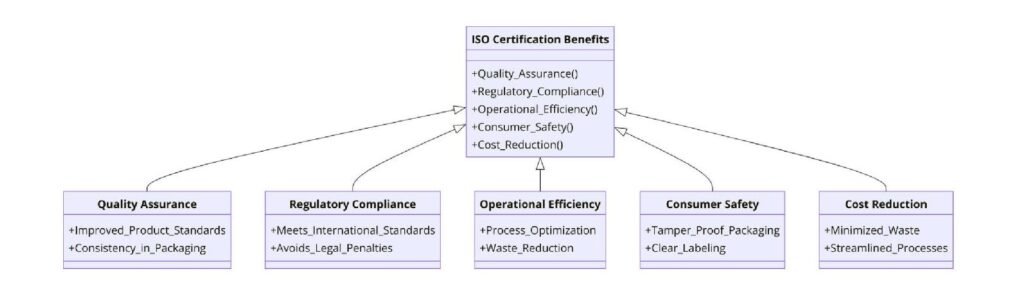
Product Safety and Compliance: ISO certifications help companies maintain high safety standards, reducing risks of contamination and mislabeling.
Market Access: With ISO certification, pharmaceutical companies can expand their market reach, as many countries require certified suppliers for regulatory approval.
Efficiency: Standardized processes minimize errors, reduce waste, and optimize production, leading to cost savings.
Customer Trust: ISO-certified companies gain a competitive edge, as customers prefer products that meet international quality standards.
Sustainability: ISO 14001 certification helps companies adopt green packaging solutions, aligning with global sustainability trends.
The pharmaceutical packaging and labeling sector is evolving rapidly. In this year, key trends include
- Sustainable Packaging: Companies are shifting to biodegradable and recyclable materials to meet environmental regulations.
- Smart Packaging & Digital Labels: QR codes and NFC technology are being integrated to provide real-time product tracking and authentication.
- Anti-Counterfeiting Measures: Advanced security features, such as holograms and tamper-evident seals, are becoming standard to prevent fake drugs from entering the supply chain.
These trends emphasize the need for pharmaceutical companies to adopt ISO standards to stay competitive and compliant.
Achieve global compliance and enhance the credibility of your pharmaceutical packaging and labeling services with ISO certification. Pacific Certifications provides independent, third-party auditing and certification to help you meet international standards!
Pacific Certifications is accredited by ABIS, in case you need support with ISO certification for your Pharmaceuticals Packing and Labelling Services business, please contact us at support@pacificcert.com or +91-8595603096.
FAQs: ISO Certifications for Pharmaceuticals Packing and Labelling Services
ISO 15378:2017 is specifically designed for primary packaging materials in pharmaceuticals. However, ISO 9001, ISO 13485, and ISO 14001 are also commonly required.
The certification process varies based on company size and compliance readiness. Typically, it takes 3-6 months, depending on the standard being pursued.
Yes, but ISO certification provides a competitive advantage and helps meet regulatory requirements in global markets.
No, Pacific Certifications is a certification body. We conduct audits and issue ISO certifications but do not provide consultancy or implementation services.
Contact support@pacificcert.com for a certification audit and compliance evaluation. Our experts will guide you through the process.
Read More at: Blogs by Pacific Certifications






