What is ISO 13485:2016 Medical Devices – Quality Management Systems?
ISO 13485:2016 outlines the requirements for a comprehensive quality management system (QMS) specific to the medical devices industry. This standard is designed to be used by organizations involved in the design, production, installation, and servicing of medical devices and related services. ISO 13485 ensures that medical devices consistently meet customer and regulatory requirements, emphasizing safety and efficacy.
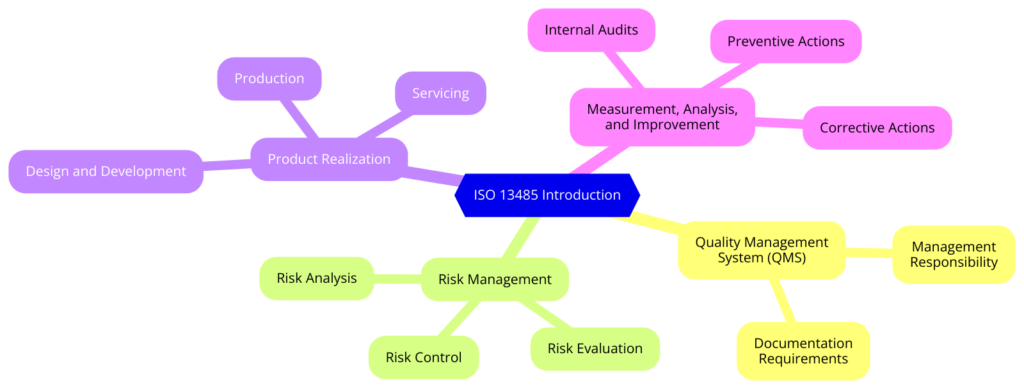
What are the Key Elements of ISO 13485:2016
Below are the key elements and requirements of ISO 13485:
- Quality Management System (QMS)
- Management Responsibility
- Resource Management
- Product Realization
- Measurement, Analysis, and Improvement
- Risk Management
- Regulatory Requirements
ISO 13485 enhances the organization’s credibility and ensures the safety and efficacy of its medical devices, thereby fostering trust among customers and regulatory bodies.
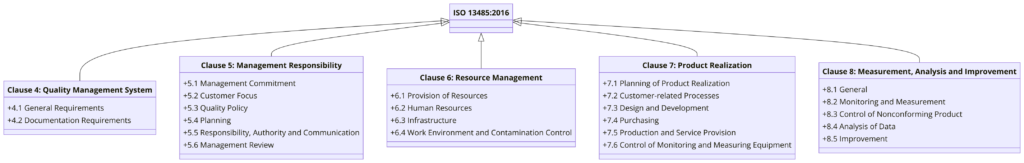
Structure of ISO 13485:2016 – Clause Wise
ISO 13485:2016 is structured into eight main clauses, each addressing different aspects of the Quality Management System (QMS) specific to the medical device industry.
Here is an overview of the clauses:
Clause 4: Quality Management System
- 4.1 General Requirements: Establish, document, implement, and maintain a QMS and continually improve its effectiveness.
- 4.2 Documentation Requirements: Define the documentation requirements, including the quality manual, control of documents, and control of records.
Clause 5: Management Responsibility
- 5.1 Management Commitment: Top management must demonstrate leadership and commitment to the QMS.
- 5.2 Customer Focus: Ensure that customer and regulatory requirements are determined and met.
- 5.3 Quality Policy: Establish a quality policy that aligns with the organization’s purpose and provides a framework for quality objectives.
- 5.4 Planning: Set quality objectives and plan the QMS to achieve them.
- 5.5 Responsibility, Authority, and Communication: Define roles, responsibilities, and authorities; ensure effective communication.
- 5.6 Management Review: Conduct regular management reviews to ensure the QMS’s continuing suitability, adequacy, and effectiveness.
Clause 6: Resource Management
- 6.1 Provision of Resources: Determine and provide the resources needed to implement and maintain the QMS.
- 6.2 Human Resources: Ensure that personnel are competent based on appropriate education, training, skills, and experience.
- 6.3 Infrastructure: Provide and maintain the necessary infrastructure to achieve product conformity.
- 6.4 Work Environment: Manage the work environment to ensure product safety and compliance.
Clause 7: Product Realization
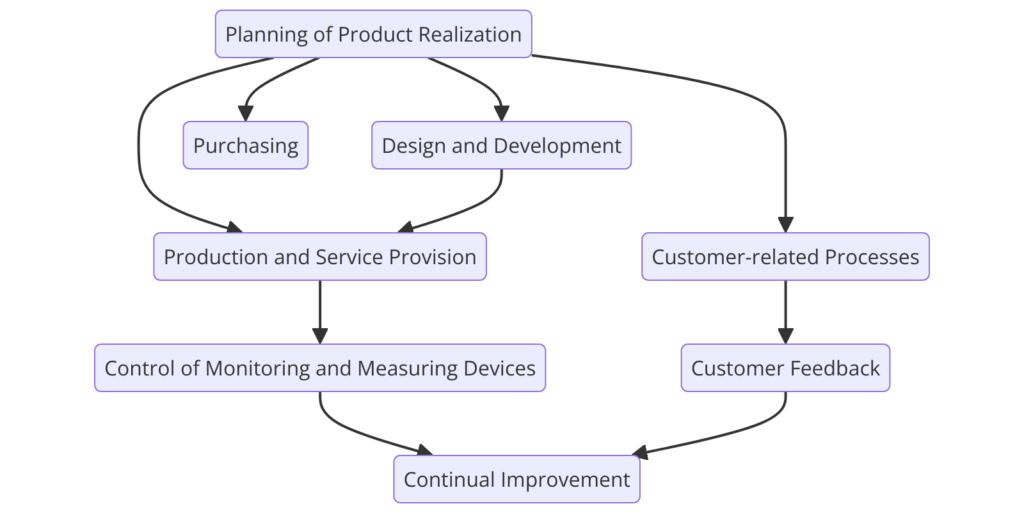
- 7.1 Planning of Product Realization: Plan and develop processes needed for product realization.
- 7.2 Customer-Related Processes: Determine and review customer requirements.
- 7.3 Design and Development: Control the design and development process, including planning, inputs, outputs, reviews, verification, validation, and changes.
- 7.4 Purchasing: Control purchasing processes to ensure that purchased products conform to specified requirements.
- 7.5 Production and Service Provision: Plan and control production and service provision, including validation of processes, control of production equipment, and monitoring and measurement activities.
- 7.6 Control of Monitoring and Measuring Equipment: Ensure that monitoring and measurement equipment is calibrated and maintained to provide accurate and reliable results.
Clause 8: Measurement, Analysis, and Improvement
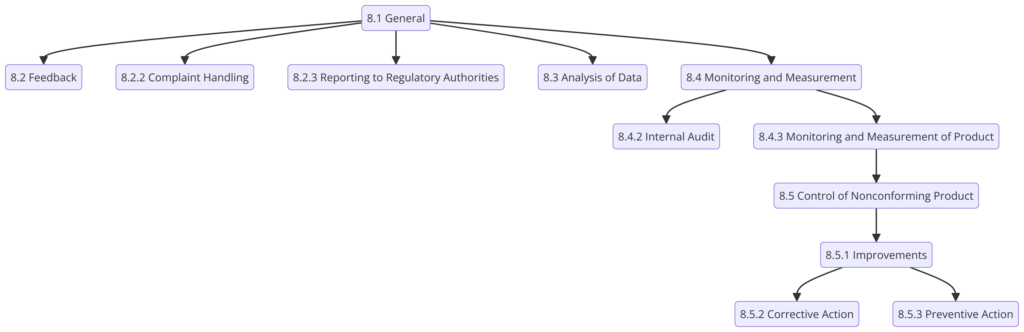
- 8.1 General: Plan and implement the monitoring, measurement, analysis, and improvement processes needed to demonstrate product conformity, ensure QMS effectiveness, and continually improve the QMS.
- 8.2 Monitoring and Measurement: Conduct regular internal audits, monitor customer satisfaction, and measure QMS processes.
- 8.3 Control of Nonconforming Product: Identify and control nonconforming products to prevent their unintended use or delivery.
- 8.4 Analysis of Data: Collect and analyze data to identify trends and opportunities for improvement.
- 8.5 Improvement: Implement corrective and preventive actions to eliminate the causes of nonconformities and continually improve the QMS.
Clause 9: Risk Management
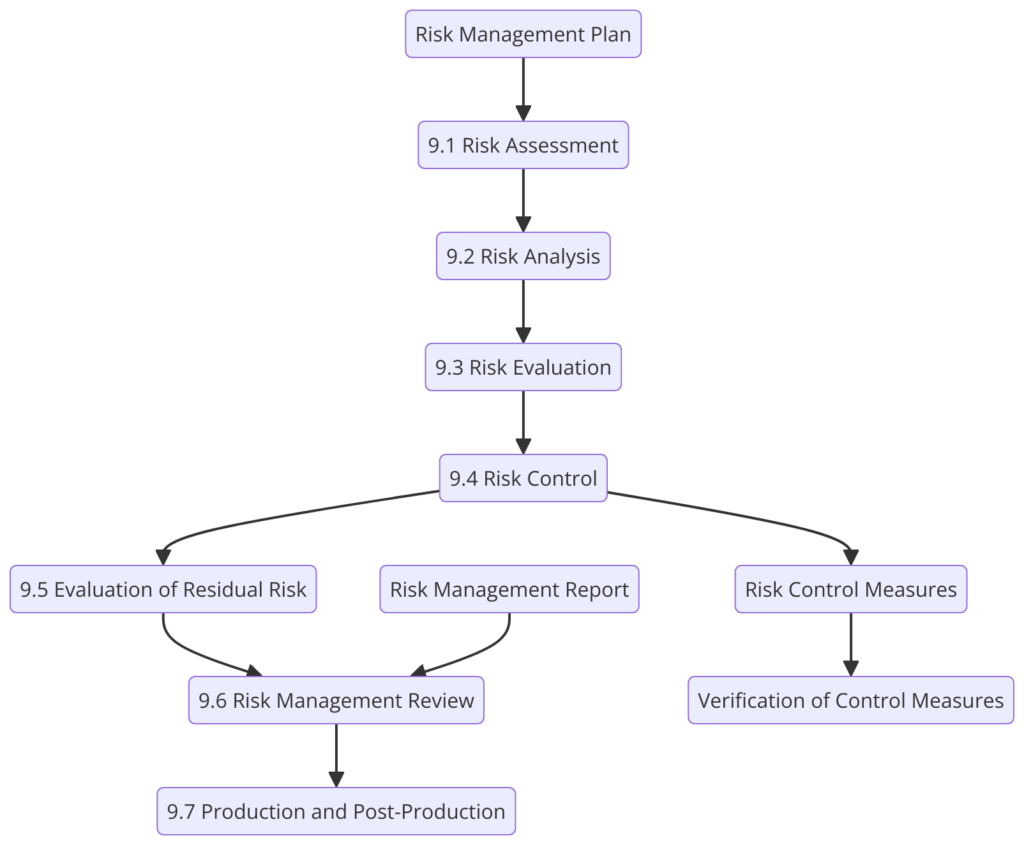
Risk management is integrated throughout the standard. Organizations are required to identify, evaluate, and control risks associated with medical devices during design, production, and post-market surveillance.
Clause 10: Regulatory Requirements
Compliance with applicable regulatory requirements is a fundamental aspect of the QMS, ensuring that medical devices meet local, national, and international regulations.
Organizations can achieve ISO 13485:2016 certification by implementing above clauses, ensuring that their quality management system is robust, effective, and compliant with industry and regulatory standards. Being ISO 13485 certified helps to enhance product quality, safety, and reliability, fostering trust among customers and regulatory bodies.
Audit Checklist for ISO 13485:2016: Clause-wise
Clause 4: Quality Management System
- Is there a documented Quality Management System (QMS)?
- Are QMS processes identified and documented?
- Are responsibilities and authorities defined?
- Are risks identified and managed?
- Is there evidence of continual improvement?
- Is there a quality manual?
- Are document control procedures in place?
- Are records controlled and maintained?
Clause 5: Management Responsibility
- Is top management demonstrating commitment to the QMS?
- Are resources being provided for the QMS?
- Are customer requirements determined and met?
- Is there a documented quality policy?
- Is the quality policy communicated and understood within the organization?
- Are quality objectives established and measurable?
- Are there plans to achieve quality objectives?
- Are responsibilities and authorities defined and communicated?
- Are effective communication processes in place?
- Are regular management reviews conducted?
- Are management review inputs and outputs documented?
Clause 6: Resource Management
- Are resources sufficient to implement and maintain the QMS?
- Are personnel competent based on education, training, skills, and experience?
- Is there a training and competency evaluation process?
- Is the infrastructure adequate to achieve product conformity?
- Is the work environment managed to ensure product safety and compliance?
Clause 7: Product Realization
- Is there planning for product realization processes?
- Are customer requirements determined and reviewed?
- Are customer communication processes in place?
- Are design and development processes controlled?
- Are design inputs, outputs, reviews, verification, validation, and changes documented?
- Are suppliers evaluated and selected based on their ability to provide products that meet requirements?
- Are purchasing processes controlled?
- Are production and service provision processes controlled?
- Are validated processes in place?
- Is production equipment maintained?
- Are monitoring and measurement activities implemented?
- Is monitoring and measurement equipment calibrated and maintained?
- Are records of calibration and maintenance kept?
Clause 8: Measurement, Analysis, and Improvement
- Are processes for monitoring, measurement, analysis, and improvement planned and implemented?
- Are internal audits conducted regularly?
- Is customer satisfaction monitored and measured?
- Are product and process characteristics monitored and measured?
- Are nonconforming products identified and controlled?
- Are corrective actions taken to prevent recurrence?
- Is data collected and analyzed to identify trends and opportunities for improvement?
- Are corrective and preventive actions implemented to eliminate the causes of nonconformities?
- Is there evidence of continual improvement of the QMS?
Clause 9: Risk Management
- Is risk management integrated into the QMS?
- Are risks identified, evaluated, and controlled throughout the product lifecycle?
Clause 10: Regulatory Requirements
- Is the QMS compliant with applicable regulatory requirements?
- Are regulatory requirements identified and addressed in the QMS processes?
This structured approach helps to identify areas of improvement and ensure that medical devices meet both customer and regulatory requirements.
Plan-Do-Check-Act (PDCA) Explained: ISO 13485:2016
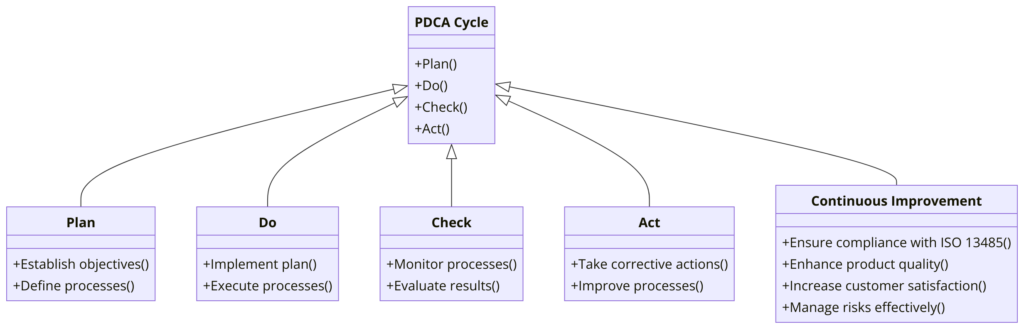
The Plan-Do-Check-Act (PDCA) cycle is a continuous improvement model that is applied to the Quality Management System (QMS) required by ISO 13485:2016. This model ensures systematic planning, implementation, monitoring, and improvement of processes to achieve higher standards of quality and compliance in the medical device industry.
ISO 13485 integrates the PDCA cycle throughout its clauses to ensure a systematic approach to managing and improving quality management systems in the medical device industry. By applying PDCA, organizations can:
- Plan: Establish a solid foundation for their QMS through careful planning and risk management.
- Do: Implement the planned activities effectively to meet quality and regulatory requirements.
- Check: Monitor and measure processes and products to ensure they meet the desired outcomes.
- Act: Continuously improve the QMS to enhance product quality, safety, and customer satisfaction.
Plan
- Identify customer and regulatory requirements.
- Conduct risk assessments to identify potential hazards and implement measures to mitigate them.
- Define measurable quality objectives aligned with the quality policy.
- Develop plans for product realization, including design and development, production, and service provision.
- Identify and allocate resources necessary to achieve quality objectives.
- Create and maintain necessary documentation, including quality manuals, procedures, and records.
Do
- Execute the planned processes for product realization.
- Ensure personnel are trained and competent to perform their tasks.
- Manage and control operations to ensure product conformity.
- Select and control suppliers to ensure that purchased products meet specified requirements.
- Develop products according to design and development plans, ensuring all requirements are met.
Check
- Monitor and measure process performance and product conformity.
- Conduct internal audits to ensure the QMS is effectively implemented and maintained.
- Collect and analyze customer feedback to assess satisfaction.
- Identify and control nonconforming products and processes.
- Analyze data to identify trends, risks, and opportunities for improvement.
Act
- Implement corrective actions to eliminate the causes of nonconformities.
- Identify and implement preventive actions to avoid potential nonconformities.
- Evaluate and enhance the effectiveness of the QMS and its processes.
- Conduct management reviews to assess QMS performance and make strategic decisions.
This cyclical process ensures that the QMS remains dynamic, responsive to changes, and focused on continual improvement, aligning with the stringent requirements of the medical device industry.
Global Trends in ISO 13485 Adoption and Benefits for Companies
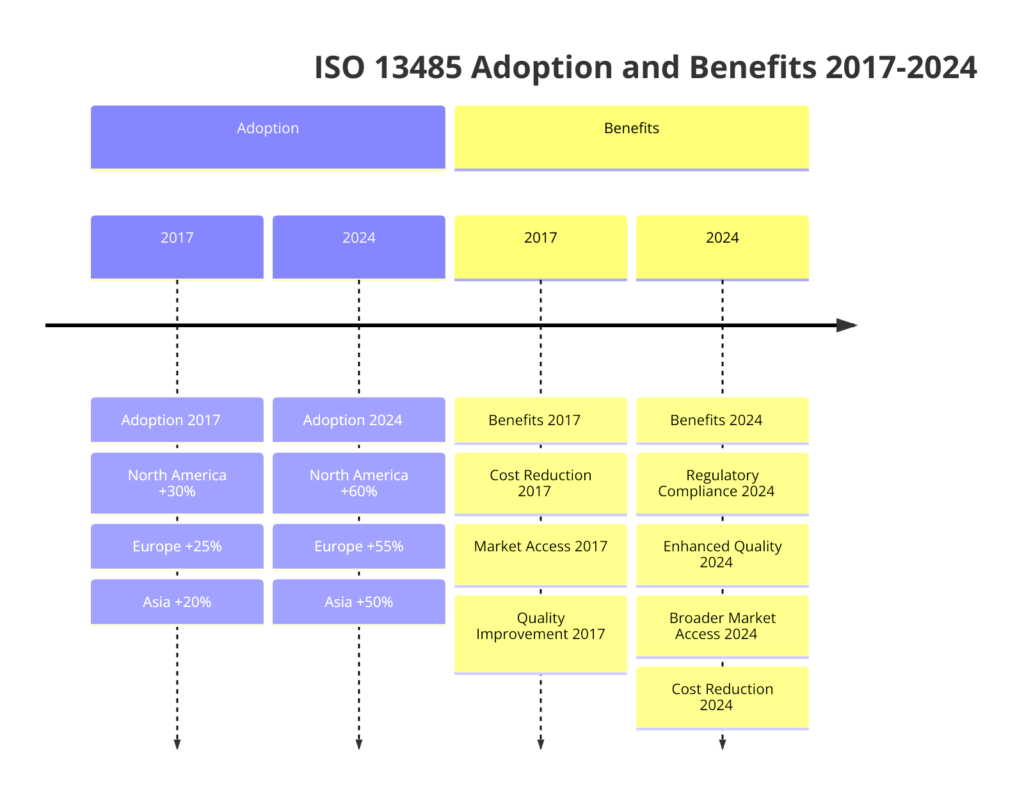
ISO 13485 standard is crucial for organizations involved in the design, production, installation, and servicing of medical devices, as well as related services. It demonstrates a company’s commitment to quality, regulatory compliance, and continuous improvement, which is essential in the highly regulated medical industry.
In recent years, the adoption of ISO 13485:2016 has seen a significant increase globally. This trend is followed by:
Many countries have integrated ISO 13485 into their regulatory frameworks for medical devices. For instance, the European Union’s Medical Device Regulation (MDR) requires manufacturers to comply with ISO 13485:2016.
With the increasing complexity of medical devices, ensuring quality and safety has become paramount. ISO 13485 provides a structured approach to maintaining high-quality standards throughout the product lifecycle.
ISO 13485 emphasizes risk management and control of production processes. Companies report that adhering to these standards has significantly enhanced their product quality and safety. Boston Scientific noted that implementing ISO 13485:2016 helped them streamline their processes and reduce defects, leading to safer and more reliable medical devices.
Compliance with ISO 13485 helps companies meet regulatory requirements more efficiently. Medtronic, a global leader in medical technology, credits ISO 13485:2016 certification for aiding in their regulatory submissions across different regions, thus speeding up the approval process and reducing time-to-market.
Market Access and Business Growth
ISO 13485 certification opens doors to international markets by meeting the quality and regulatory expectations of various countries. Zimmer Biomet, a prominent orthopedic device manufacturer, expanded its market presence significantly after obtaining ISO 13485:2016 certification, leading to increased sales and market share.
By ensuring high-quality products and consistent processes, companies can achieve higher customer satisfaction. Philips Healthcare reported that ISO 13485:2016 certification led to improved customer feedback and loyalty, contributing to long-term business relationships and repeat business.
ISO 13485 Certification Requirements: Implementing ISO 13485:2016
Implementing ISO 13485:2016 involves a comprehensive approach to ensuring that an organization’s processes meet regulatory and customer requirements consistently. Below is a detailed overview of the key requirements and steps involved in implementing ISO 13485.
What are the Key Requirements of ISO 13485:2016
Quality Management System (QMS)
- Establish, document, implement, and maintain a QMS.
- Maintain a quality manual, documented procedures, and records necessary to ensure effective planning, operation, and control of processes.
Management Responsibility
- Demonstrate top management’s commitment to the development and implementation of the QMS and continuously improving its effectiveness.
- Ensure that customer requirements are determined and met.
- Establish a quality policy that is appropriate to the purpose of the organization.
- Set quality objectives and plan the QMS to achieve them.
- Define roles, responsibilities, and authorities.
- Conduct regular reviews of the QMS.
Resource Management
- Determine and provide the resources needed.
- Ensure personnel are competent.
- Provide and maintain the necessary infrastructure.
- Manage the work environment to ensure product safety and compliance.
Product Realization
- Plan and develop processes needed for product realization.
- Determine customer requirements and ensure effective communication.
- Implement controlled design and development processes.
- Control the purchasing processes to ensure conformity.
- Plan and control production and service provision.
- Ensure that monitoring and measurement equipment is calibrated and maintained.
Measurement, Analysis, and Improvement
- Plan and implement monitoring, measurement, analysis, and improvement processes.
- Conduct internal audits and monitor customer satisfaction.
- Identify and control nonconforming products.
- Collect and analyze data to identify trends and opportunities for improvement.
- Implement corrective and preventive actions and continually improve the QMS.
Steps to Implement ISO 13485:2016
- Gap Analysis
- Project Planning
- Training
- Documentation
- Process Implementation
- Internal Audits
- Management Review
- Corrective and Preventive Actions
- External Audit
- Certification
By meeting these requirements, organizations can achieve certification, demonstrating their commitment to quality and regulatory compliance in the medical device industry.
For more detailed information on implementing ISO 13485:2016 and its benefits, you can refer to International Organization for Standardization.
Steps to achieve ISO 13485:2016 Certification
Achieving ISO 13485:2016 certification involves a structured approach to implementing a quality management system (QMS) specific to the medical devices industry. Here are the detailed steps to achieve this certification:
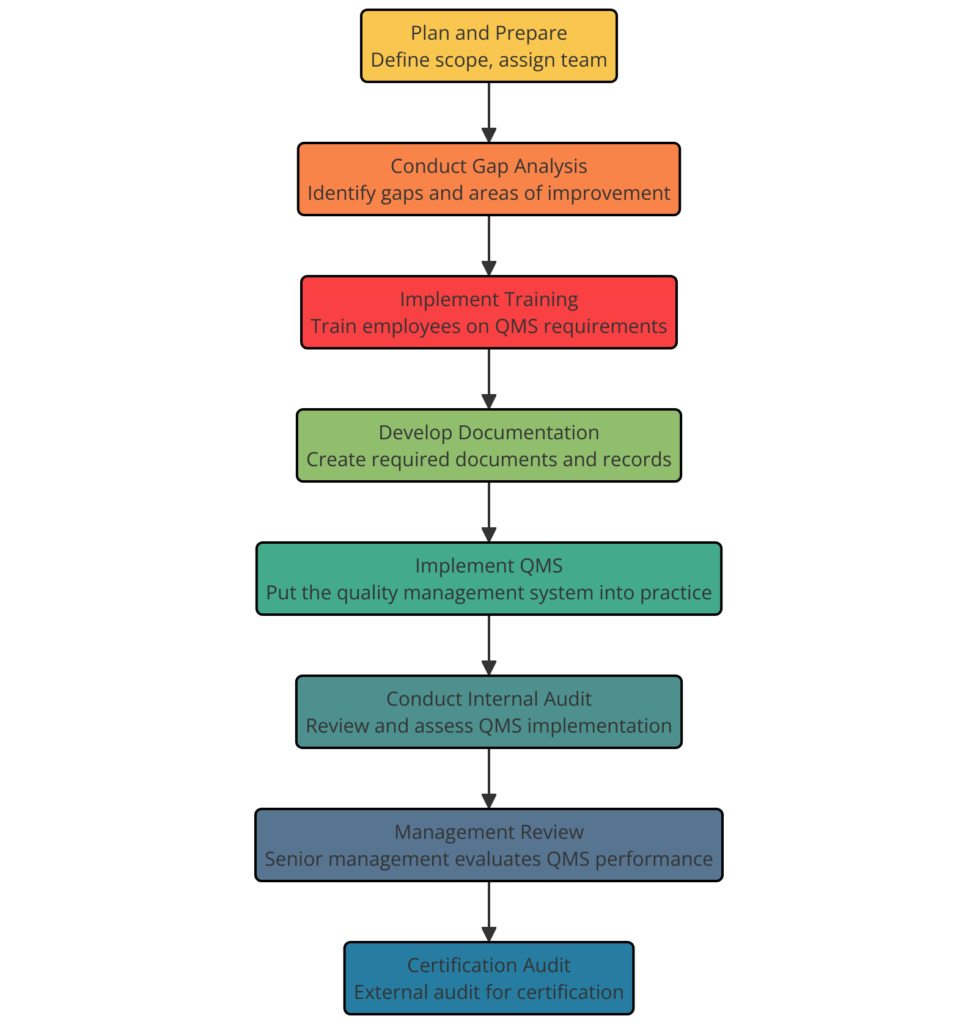
- Understand ISO 13485:2016 Requirements
- Conduct a Gap Analysis
- Develop an Implementation Plan
- Establish a Quality Management System (QMS)
- Implement the QMS
- Internal Audits
- Management Review
- Pre-Assessment
- Certification Audit: Choose an accredited certification body to conduct the certification audit.
- Stage 1 Audit (Documentation Review): The certification body will review your QMS documentation to ensure it meets ISO 13485:2016 requirements.
- Stage 2 Audit (On-Site Audit): The certification body will perform an on-site audit to verify that your QMS is effectively implemented and complies with the standard.
- Achieving Certification
- Maintaining Certification
By following these steps, organizations can effectively implement ISO 13485 and achieve certification, ensuring they meet the necessary quality and regulatory requirements for medical devices.
What are the Benefits of ISO 13485:2016 Certification
ISO 13485:2016 certification offers several advantages for companies in the medical devices industry. These benefits include improved regulatory compliance, enhanced product quality, increased customer satisfaction, and expanded market access. Below are some of the key benefits, illustrated with case studies and external references where companies have described the positive impact of achieving ISO 13485 certification.
Common benefits are:
- Regulatory Compliance: Ensures adherence to international medical device regulations.
- Risk Management: Enhances risk management and mitigation processes.
- Quality Improvement: Promotes continuous improvement in product quality and safety.
- Customer Satisfaction: Increases customer confidence and satisfaction.
- Market Access: Facilitates entry into global markets with recognized certification.
- Operational Efficiency: Streamlines processes and reduces waste.
- Competitive Advantage: Provides a competitive edge in the medical device industry.
- Supplier Confidence: Builds trust with suppliers and stakeholders.
- Traceability: Improves traceability and documentation throughout the product lifecycle.
- Legal Protection: Reduces liability risks through stringent quality management.
ISO 13485:2016 certification ensures that a company’s quality management system (QMS) meets international regulatory requirements for medical devices, facilitating compliance with various national and regional regulations.
Below are some case studies from some popular companies explaining how ISO 13485 helped them:
- Siemens Healthineers: Siemens Healthineers achieved ISO 13485 certification to streamline their regulatory compliance processes across multiple countries. This certification helped them meet the stringent requirements of the European Union Medical Device Regulation (MDR) and other global regulatory standards, thereby reducing the complexity and cost of regulatory compliance.
- Medtronic leveraged ISO 13485 certification to enhance their product development processes. The certification helped them identify and mitigate risks early in the design phase, resulting in fewer product recalls and higher customer satisfaction.
- Johnson & Johnson reported that achieving ISO 13485:2016 certification improved their customer satisfaction scores by ensuring that their products consistently met customer expectations and regulatory requirements. This, in turn, helped them retain existing customers and attract new ones.
- Philips Healthcare used ISO 13485:2016 certification to gain access to new markets in Asia and Europe. The certification provided a competitive edge, enabling them to enter markets that require strict regulatory compliance for medical devices.
- Becton Dickinson (BD): achieved significant operational efficiencies through ISO 13485 certification. By standardizing their QMS processes across different facilities, they reduced variability and improved coordination, leading to cost savings and faster time-to-market for new products.
- GE Healthcare utilized ISO 13485:2016 certification to implement a robust risk management framework. This proactive approach allowed them to identify potential issues before they became critical, thus reducing the likelihood of product failures and enhancing patient safety.
- Boston Scientific’s ISO 13485 certification helped them standardize supplier quality agreements and improve communication with their suppliers. This resulted in higher quality inputs and more reliable supply chain performance.
What are the Difference between ISO 9001:2015 and ISO 13485:2016- Medical Devices – Quality Management Systems?
ISO 9001:2015 and ISO 13485:2016 are both international standards related to quality management systems, but they have some key differences, particularly in their focus and scope. Here are the main differences between ISO 9001:2015 and ISO 13485:2016:
Focus and Industry Specificity:
ISO 9001:2015: This standard provides a generic framework for quality management systems applicable to any organization, regardless of its industry or sector. It emphasizes customer satisfaction, continuous improvement, and the adoption of a process-based approach to quality management
ISO 13485:2016: This standard is specifically tailored for organizations involved in the design, development, production, installation, and servicing of medical devices. So, It incorporates additional requirements specific to the medical device industry and emphasizes product safety, regulatory compliance, and risk management
Regulatory Compliance:
ISO 9001:2015: While ISO 9001:2015 promotes compliance with applicable statutory and regulatory requirements, it does not provide specific requirements for regulatory compliance in any particular industry
ISO 13485:2016: It is aligned with regulatory requirements specific to the medical device industry. It includes specific provisions to address the unique regulatory environment, such as documentation and record-keeping requirements, risk management, and product traceability
Risk Management:
ISO 9001:2015: The standard requires organizations to identify and address risks and opportunities that could affect the conformity of products and the effectiveness of the quality management system. However, it does not provide detailed guidance on risk management processes.
ISO 13485:2016: It places a greater emphasis on risk management throughout the product lifecycle. It requires organizations to establish and maintain processes for risk management, including the identification, assessment, mitigation, and monitoring of risks associated with medical devices.
Product Realization:
ISO 9001:2015: It provides general requirements for product realization, covering the design and development, production, and service provision processes. However, it does not include industry-specific requirements
ISO 13485:2016: It includes more specific requirements for product realization in the medical device industry. It addresses activities such as design and development controls, validation and verification, control of production and service provision, and the establishment of installation and servicing procedures
Post-Market Considerations:
ISO 9001:2015: It does not explicitly address post-market activities, such as complaint handling, adverse event reporting, and vigilance activities
ISO 13485:2016: It includes specific requirements for post-market activities in the medical device industry. It requires organizations to establish processes for complaint handling, reporting adverse events, and implementing corrective and preventive actions related to product performance and safety
There are differences between ISO 9001:2015 and ISO 13485:2016, they also share some common elements, such as the importance of customer focus, process approach, continuous improvement, and the involvement of top management.
Organizations in the medical device industry often choose ISO 13485 to meet both regulatory requirements and demonstrate their commitment to quality management specific to medical devices, while ISO 9001:2015 can be more widely applicable across industries.
Who needs ISO 13485:2016-Medical devices – Quality Management Systems?
ISO 13485:2016 is designed for organizations involved in the design, development, production, installation, and servicing of medical devices. It is applicable to a wide range of entities within the medical device industry, including:
Manufacturers: Companies involved in the manufacturing of medical devices, including those that design, develop, produce, assemble, package, and label medical devices.
Distributors: Organizations engaged in the distribution of medical devices, including wholesalers, distributors, and importers.
Service Providers: Companies providing services related to medical devices, such as installation, maintenance, repair, and calibration.
Contract Manufacturers: Organizations that manufacture medical devices on behalf of other companies under contract.
Component and Material Suppliers: Suppliers of components, raw materials, and substances used in the production of medical devices.
Testing and Calibration Laboratories: Laboratories providing testing, calibration, and analysis services for medical devices.
Regulatory Bodies: Organizations responsible for regulating and overseeing the medical device industry.
Contract Research Organizations: Organizations involved in research and development activities related to medical devices.
Sterilization Service Providers: Entities providing sterilization services for medical devices.
Other Organizations in the Supply Chain: Entities involved in the medical device supply chain, including logistics providers, storage facilities, and transporters.
Therefore, ISO 13485:2016 is applicable to various organizations within the medical device industry, the specific requirements and scope may vary depending on the organization’s size, complexity, and the nature of its activities.
Organizations seeking ISO 13485 certification should carefully assess the standard’s requirements and ensure that they meet the necessary criteria for their specific role within the medical device industry
Pacific Certifications is accredited by ABIS, Click here to apply for ISO 13485:2016 or get in touch with us at +91-8595603096 or support@pacificcert.com
Suggested Certifications –









