ISO Certifications for Pharmaceuticals & Life Sciences Industry

The pharmaceuticals and life sciences industry supports global health through drug development clinical research and diagnostics. It includes R&D labs manufacturing units, biotech startups and diagnostic service providers. The work spans drug discovery formulation testing packaging and delivery.
Each segment works under different pressures. Drug makers must meet global quality expectations. Biotech labs focus on cleanroom conditions and sterile environments. Life sciences firms handle supply chains data logs and product approvals. They also deal with rapid innovation cycles and changing global requirements. This creates a need for stable and clear processes.
ISO certifications help companies manage this complexity. These standards apply to quality health and safety environmental care and information security. They guide companies to build consistent systems that reduce risks and improve control.
To begin your ISO journey or schedule an audit contact us at support@pacificcert.com
Why ISO Certifications Matter for Pharmaceuticals & Life Sciences?
This Pharmaceuticals & Life Sciences Industry works under tight rules. Every process—from research and formulation to packaging and delivery—must meet defined safety and quality conditions. Failures can lead to product recalls halted clinical trials or even harm to patients. Companies must manage data integrity lab practices sterilization packaging logistics and employee safety while also facing pressure to innovate fast.
Workplace risks such as chemical exposure lab accidents and repetitive tasks are common. Data breaches or errors in electronic records can lead to compliance failure. Sustainability is also a growing concern with firms being asked to cut waste manage energy and use ethical sourcing. ISO certifications provide guidance on building consistent processes in all these areas. They allow companies to maintain global acceptance, build trust and keep operations traceable and controlled. In recent years there has also been a rising demand for transparency in supply chain sourcing and ethical testing practices. More countries are linking certification to licensing and government funding. Companies now use ISO certification to gain an edge in investor relations contract approvals and cross-border regulatory recognition. The result is that ISO is no longer seen as optional but as part of basic business readiness.
Applicable ISO standards for Pharmaceuticals & Life Sciences
ISO 9001 – Quality Management Systems
ISO 9001 helps pharma and biotech companies manage quality at every stage—from raw material inspection to packaging and final release. It supports documentation batch control process validation and customer feedback tracking.
ISO 13485 – Medical Devices Quality Management
For life sciences companies involved in diagnostic kits surgical tools or drug-delivery devices ISO 13485 outlines quality standards specific to medical devices. It supports sterile production labeling traceability and product lifecycle management.
ISO 14001 – Environmental Management Systems
This standard is useful for companies aiming to reduce chemical waste manage emissions or build eco-friendly facilities. It supports structured environmental monitoring and planning.
ISO 45001 – Occupational Health & Safety
Workplace safety is vital in labs and production units. ISO 45001 helps organizations identify risks, prevent accidents and support employee well-being through safe practices and incident tracking.
ISO 27001 – Information Security Management
With growing dependence on digital lab records clinical trial data and cloud-based research tools ISO 27001 helps in protecting sensitive information from loss leaks or cyber threats.
ISO 14971 – Risk Management for Medical Devices
ISO 14971 is used by pharmaceutical and life sciences companies involved in the design manufacture and evaluation of medical devices. It provides a structured approach to identifying and managing risks related to product safety usability and performance throughout the lifecycle.
What are ISO Certification requirements for Pharmaceuticals & Life Sciences?
To achieve ISO certification, organizations in the Pharmaceuticals & Life Sciences Industry need to take structured steps across quality safety or data systems.. These steps make sure the processes work as intended and stay reliable over time.
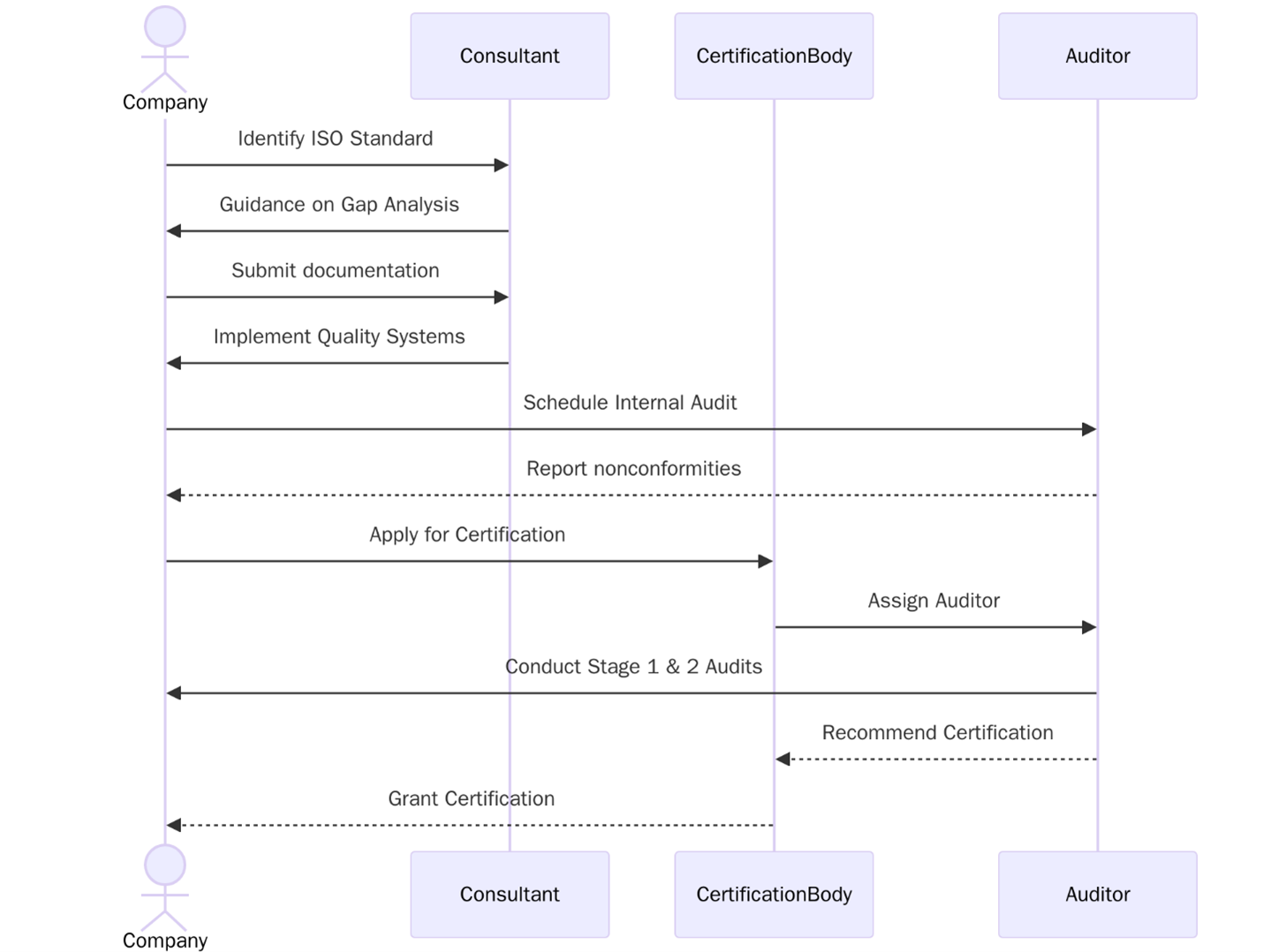
- Conduct a gap analysis against the chosen ISO standard
- Appoint a responsible team or project leader
- Document existing policies SOPs and workflows
- Create an implementation plan with clear timelines
- Train internal teams on standard-specific topics
- Monitor process performance through audits and reviews
- Identify and manage risks in operations and supply chains
- Maintain records of training inspection and corrective actions
- Conduct internal audits to check system readiness
- Undergo a Stage 1 audit by a certification body
- Complete a Stage 2 audit to confirm system implementation
For companies in Pharmaceuticals & Life Sciences Industry meeting ISO requirements is not just a process task but part of day-to-day control and safety. These standards are widely accepted in supplier approvals public tenders and customer evaluations. By following specific steps from planning to auditing companies can build systems that stay reliable in real work conditions.
What are the benefits of ISO Certifications for Pharmaceuticals & Life Sciences?
ISO certifications bring reliability to how life sciences companies manage operations and risks.. They also make it easier to pass audits meet client expectations and run safe production and research units.
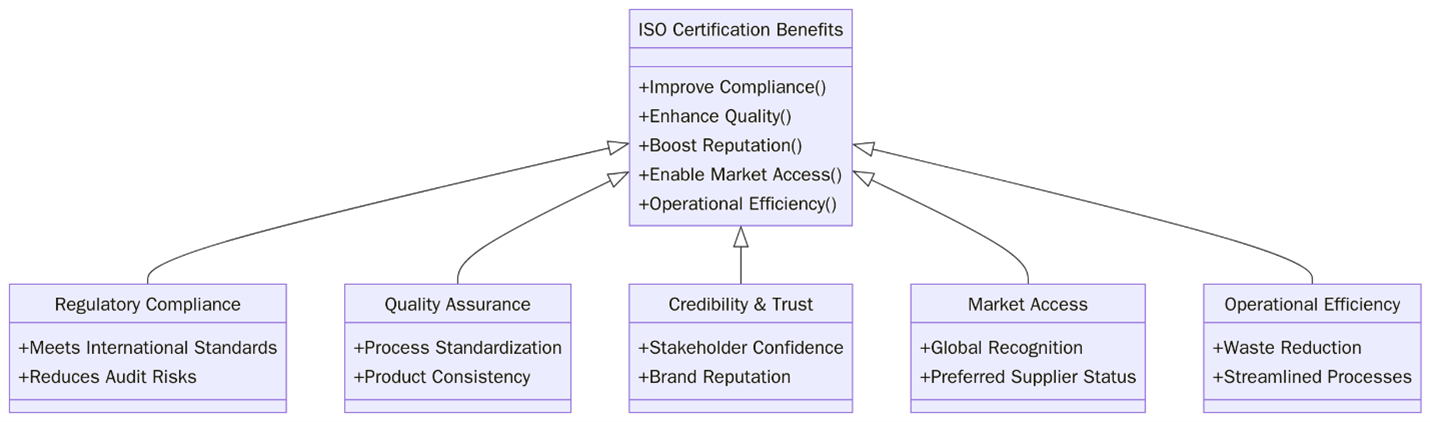
- Improved product quality
- Stronger audit readiness
- Better control of contamination risks
- Higher supply chain traceability
- Structured training programs
- Improved incident tracking and response
- Increased customer trust
- Lower non-conformance rates
- Better equipment calibration records
- Data protection and access control
- Clearer documentation and SOP control
- Improved lab and production safety
ISO certifications allow businesses in this space to manage technical processes more consistently and improve the way they track and report issues. Whether it’s for labs research centers or drug manufacturers these benefits go beyond quality control. They impact long-term partnerships staff training supplier trust and even investor interest.
ISO Certification Trends in Pharmaceuticals & Life Sciences: 2020 vs. 2025
ISO Standard | 2020 Adoption Rate | 2025 Projected Rate | Key Growth Drivers |
ISO 13485 | 58% | 76% | Medical device growth remote health technologies |
ISO 9001 | 71% | 85% | Global sourcing quality transparency |
ISO/IEC 27001 | 33% | 64% | Data security in clinical systems and trials |
How can Pacific Certifications help?
Pacific Certifications audits companies across the pharmaceutical and life sciences sectors. Our role is to evaluate management systems through impartial accredited processes.
- We conduct document and process reviews to check readiness
- We offer flexible audit scheduling across different locations
- We provide combined audits for ISO 9001 13485 and 14001
- We guide clients on non-conformities and audit findings
- We support surveillance and re-certification audits as per timelines
We do not provide consultancy services. Our focus is on impartial value-based audits.
ISO Training and Courses
- Internal Auditor Training: Helps employees conduct internal audits for self-assessment and improvement readiness
- Lead Auditor Training: Prepares participants to lead audits on behalf of certification bodies or internal teams
- Awareness and Implementation Workshops: Introductory sessions on ISO requirements, system design, and practical steps
Pacific Certifications provides accredited training programs. If your organization is looking for ISO 9001, ISO 13485 (Medical Devices), ISO 45001, and ISO 14001 training, our team is equipped to help you. Contact us at support@pacificcert.com.
FAQ: ISO Certifications for Pharmaceuticals & Life Sciences
Which standard is best for biotech labs working on diagnostics?
ISO 13485 is ideal if the lab is developing or handling medical devices or test kits.
Can small or mid-sized companies apply for ISO certifications?
Yes. Certification is available to companies of all sizes including MSMEs.
How long is the ISO certification valid?
Most ISO certifications remain valid for three years with annual surveillance audits.
Does Pacific Certifications assist with implementation?
No. We do not offer implementation support. Our work is limited to auditing and certification.
Can multiple ISO standards be audited together?
Yes. We offer combined audits such as ISO 9001 with ISO 14001 or ISO 13485 depending on your scope.
Ready to get ISO certified?
Contact Pacific Certifications to begin your certification journey today!
Suggested Certifications –
Read more: Pacific Blogs




