What is ISO 374-1:2024?

Chemical and biological hazards are widespread across industries ranging from healthcare and laboratories to petrochemicals, agriculture, and manufacturing. Workers handling corrosive liquids, toxic substances, or infectious agents require reliable protective equipment that can withstand direct contact and provide long-term safety. ISO 374-1:2024 establishes the international standard for protective gloves against dangerous chemicals and micro-organisms. It sets out the design, performance, and testing criteria that gloves must meet before they are accepted as compliant.
This standard not only safeguards individuals but also supports organizations in meeting international health and safety requirements. With increasing global attention on workplace safety and hygiene, ISO 374-1 has become a benchmark for industries where chemical and biological exposure is a daily risk.
To learn how your organization can adopt ISO 374-1 compliant safety equipment, contact support@pacificcert.com.
What is the Purpose of ISO 374-1?
The primary purpose of ISO 374-1:2024 is to ensure that protective gloves provide consistent and reliable protection against hazardous chemicals and micro-organisms. Ordinary gloves may fail under extended exposure to corrosive or infectious materials, potentially resulting in severe health risks, contamination or workplace accidents. By defining stringent testing methods and resistance classifications, the standard gives manufacturers a clear framework to produce gloves that can withstand specific hazardous conditions.
At the same time, employers gain confidence when selecting personal protective equipment (PPE) that meets recognized benchmarks. ISO 374-1 also ensures international comparability of safety equipment, allowing organizations to select certified gloves without doubt about their performance level. Its objective is to protect the health and safety of workers while ensuring consistency in global safety practices.
Scope and Applicability
ISO 374-1:2024 applies to protective gloves designed to safeguard users against hazardous chemicals and dangerous micro-organisms. The scope includes performance requirements related to chemical penetration, degradation and permeation resistance. It also specifies classification systems, glove marking and user instructions.
This standard is applicable in industries such as healthcare and pharmaceuticals, where gloves protect against infectious fluids and microorganisms. It is also applicable in chemical processing, petrochemicals, laboratories, where exposure to corrosive substances is routine, agriculture, where workers encounter pesticides, fertilizers and biological agents. Apart from this, it is used in food manufacturing and cleaning services, where disinfectants and cleaning chemicals are widely used.
ISO 374-1 is globally recognized and complements other safety frameworks such as EN 374 in Europe. Its application is not limited to large corporations – small and medium businesses handling chemicals also benefit by adopting gloves tested under this standard.
Key Definitions
- Permeation: Movement of a chemical through glove material at a molecular level.
- Penetration: Passage of a chemical or micro-organism through seams or imperfections.
- Degradation: Physical change in glove material when exposed to a chemical.
- Breakthrough Time: Time taken for a chemical to pass completely through glove material.
- Micro-organisms: Bacteria, viruses, and fungi that may pose infection risks.
Clause-wise structure of ISO 374-1:2024
| Clause | Description |
| Scope | Defines objectives, boundaries, and intended application. |
| Normative References | Lists referenced standards necessary for implementation. |
| Terms and Definitions | Provides standard terminology. |
| Requirements | Outlines performance criteria for chemical resistance and micro-organism protection. |
| Testing | Details laboratory test methods for permeation, penetration, and degradation. |
| Classification | Establishes classification of gloves based on protection level. |
| Marking and Labelling | Specifies glove marking with pictograms and relevant codes. |
| Information for Users | Provides guidance on usage, limitations, inspection, and disposal. |
What are the Requirements of ISO 374-1?
Before listing requirements, it is important to note that ISO 374-1 emphasizes both chemical resistance and biological protection. Gloves must undergo rigorous testing to validate their safety levels. Below are the key requirements:
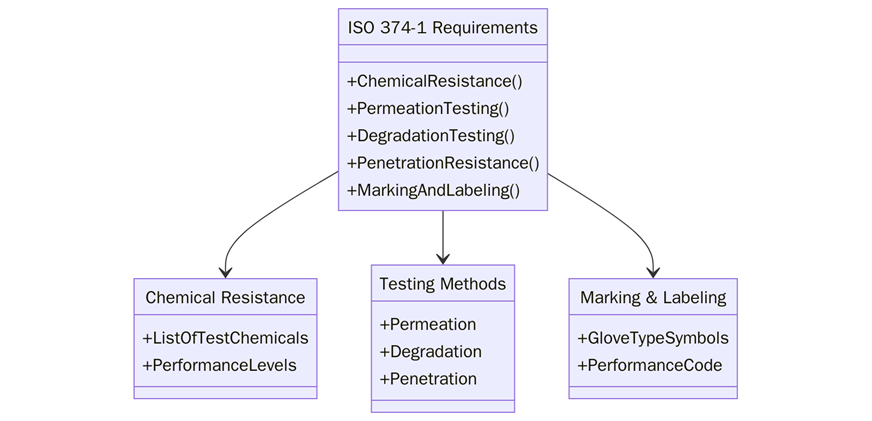
- Gloves must pass permeation resistance tests, with classification from level 1 to 6 depending on breakthrough times.
- Materials must resist chemical degradation and maintain structural integrity.
- Gloves must undergo penetration testing to ensure no leakage through seams or pinholes.
- Gloves are classified into three types (A, B, C) depending on the number of chemicals they protect against.
- Gloves must provide verified protection against micro-organisms through penetration tests.
- Clear marking and labelling must indicate chemical resistance type, tested substances, and performance level.
- User instructions must include guidelines for storage, inspection, and disposal.
- Gloves must be tested across a defined list of reference chemicals such as sulphuric acid, sodium hydroxide, and others.
- Manufacturers must provide a declaration of conformity to ISO 374-1.
What are the Benefits of ISO 374-1?
Adopting ISO 374-1 compliant gloves offers significant advantages. Beyond physical protection, it strengthens confidence in workplace safety systems. Below are the key benefits:
- Protection against hazardous chemicals reduces risks of burns, poisoning, or skin irritation.
- Barrier against micro-organisms helps prevent infections, particularly in healthcare and laboratory settings.
- Standardized classification allows employers to easily select the right gloves for specific hazards.
- Extended durability of certified gloves reduces frequent replacements and cost over time.
- Improved worker confidence as employees know they are protected by internationally tested equipment.
- Regulatory alignment supports compliance with international health and safety requirements.
- Market access for manufacturers as ISO 374-1 compliance is globally recognized.
- Insurance and liability benefits as organizations using certified PPE can show risk reduction.
- Compatibility with other PPE ensures seamless integration in occupational safety systems.
- Support for sustainability as modern glove materials tested under ISO 374-1 often have improved environmental resistance.
The global demand for protective gloves against chemicals and micro-organisms is rising sharply. Growth is fuelled by stricter occupational safety laws, increased healthcare requirements, and heightened awareness following global pandemics. Europe, North America and Asia-Pacific are leading markets, with significant growth in sectors like pharmaceuticals, petrochemical and food safety. Recent trends include the development of thinner yet more resistant gloves, biodegradable glove materials and integration of smart technologies that track glove wear and usage. Organizations adopting ISO 374-1 certified gloves not only improve safety but also strengthen their market position by meeting international procurement standards.
Certification Process for ISO 374-1
Certification involves several steps, each ensuring that gloves meet the strict criteria of ISO 374-1.
- Gap Assessment – Manufacturers or organizations review current glove specifications against ISO 374-1.
- Selection of Accredited Lab – Testing is carried out in ISO/IEC 17025 accredited laboratories.
- Testing and Verification – Gloves undergo permeation, penetration, and degradation testing.
- Classification Assignment – Gloves are categorized as Type A, B, or C depending on performance.
- Marking and Documentation – Gloves must carry standard pictograms, performance codes, and manufacturer details.
- Integration into Safety Programs – Employers update occupational health and safety systems to include ISO 374-1 gloves.
- Training – Workers receive training on correct usage, storage, and disposal.
- Audit and Surveillance – Periodic audits ensure ongoing compliance.
What is the Certification Timeline?
The timeline for ISO 374-1 certification depends on whether you are a manufacturer or an employer. For manufacturers, design, testing, and certification can take 6–12 months depending on the number of glove models tested. For employers, integrating ISO 374-1 gloves into operations typically takes 2–3 months, including procurement, training, and policy updates. Large organizations with multiple sites may require up to 6 months due to logistics and staff coverage.
What is the Certification Cost of ISO 374?
The cost of ISO 374-1 certification varies. For manufacturers, costs include lab testing, product redesign, documentation, and certification fees, which may be significant depending on product variety. Employers incur costs primarily in procurement of certified gloves, training, and audits. While these investments can be high, they are balanced by reduced workplace accidents, compliance with tender requirements, and lower insurance premiums.
How Pacific Certifications Can Help?
Pacific Certifications supports organizations and manufacturers in aligning with ISO 374-1:2024 by providing:
- Gap assessments to identify compliance gaps.
- Guidance on glove testing and classification.
- Support with marking, documentation, and labelling.
- Third-party audits for certification.
- Training programs for safe glove use and inspection.
For assistance with ISO 374-1 certification, contact support@pacificcert.com
Training and Courses
Training guides employees to handle and wear gloves safely. Key training options include:
- Lead Auditor Training– For professionals auditing glove compliance to ISO 374-1.
- Lead Implementer Training– For organizations implementing certified glove usage.
- Internal Auditor Training – For staff monitoring ongoing compliance.
Pacific Certifications provides accredited training programs. Contact support@pacificcert.com for details.
Frequently Asked Questions (FAQs)
- What is ISO 374-1:2024?
ISO 374-1 is the international standard for protective gloves against hazardous chemicals and micro-organisms.
- How are gloves tested under ISO 374-1?
They undergo permeation, penetration, and degradation testing against reference chemicals.
- What are glove classifications under ISO 374-1?
Type A gloves protect against at least six chemicals, Type B against three, and Type C against one.
- Can ISO 374-1 gloves be used in healthcare?
Yes, they are widely used in hospitals and laboratories for protection against chemicals and pathogens.
- How often should gloves be replaced?
Gloves should be replaced after visible damage, contamination, or as per manufacturer guidelines.
- Are ISO 374-1 gloves suitable for food handling?
Yes, if they are tested against relevant cleaning and disinfectant chemicals.
- What industries benefit most from ISO 374-1 gloves?
Healthcare, chemicals, agriculture, pharmaceuticals, and cleaning services.
- Is ISO 374-1 certification mandatory?
While not mandatory worldwide, many industries and governments require it for procurement.
- How long does certification take?
6–12 months for manufacturers, 2–3 months for employers integrating usage.
- Where can I get certified training?
Pacific Certifications offers accredited ISO 374-1 training programs.
Ready to get ISO ISO 374 certified?
Contact Pacific Certifications to begin your certification journey today!
Suggested Certifications –
Read more: Pacific Blogs






