What is ISO 22716?

ISO 22716 provides internationally recognized Good Manufacturing Practices (GMP) for the cosmetic industry, focusing on the production, control, storage and shipment of cosmetic products. The standard offers practical guidelines to ensure that products are consistently manufactured and controlled to meet quality and hygiene standards appropriate for their intended use.
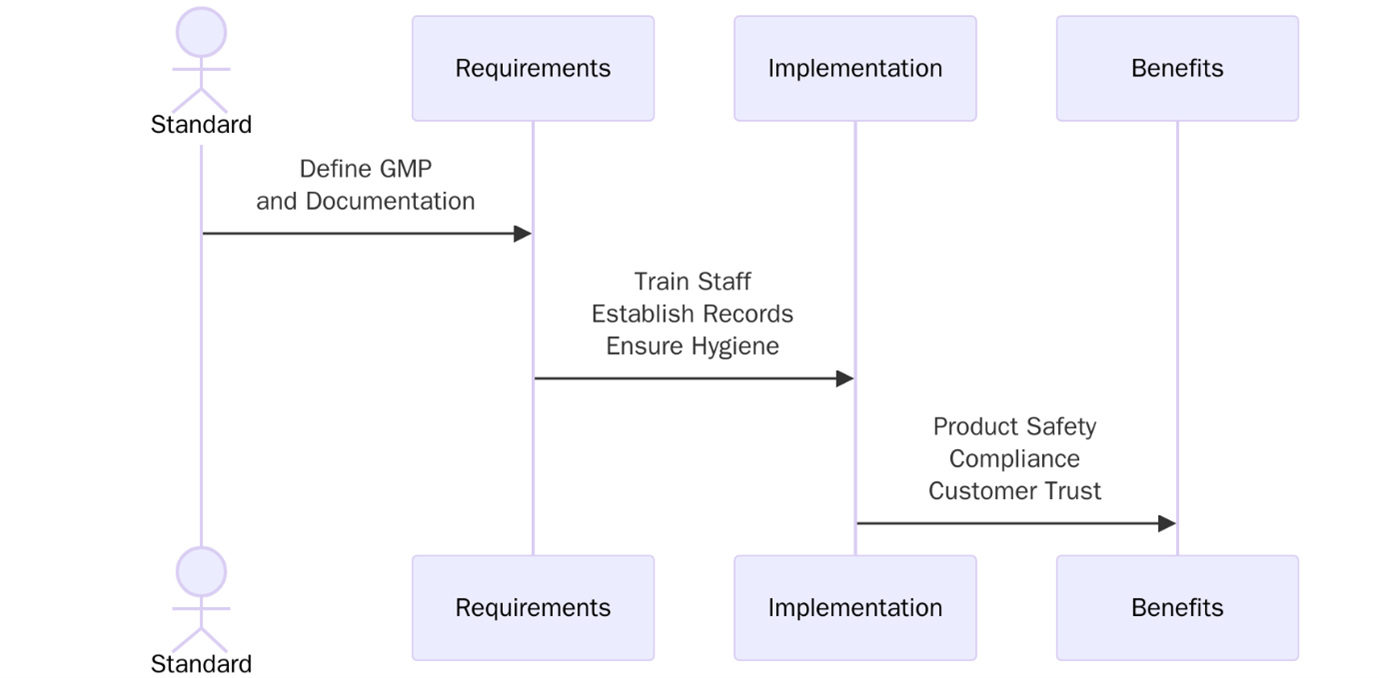
This standard bridges the gap between regulatory requirements, such as those from the EU Cosmetic Regulation (EC) No 1223/2009, US FDA, and ASEAN Guidelines, and operational practices in manufacturing environments. ISO 22716 is industry-specific, tailored exclusively for cosmetics manufacturers, brand owners and packaging suppliers.
Need help implementing GMP practices for your cosmetic manufacturing process? Contact us at support@pacificcert.com!
Scope and Applicability
ISO 22716 is applicable to:
- Cosmetic product manufacturers
- Contract manufacturers and private labelers
- Cosmetic importers and exporters
- Third-party cosmetic packaging facilities
- Warehouses and distributors handling cosmetic goods
- Regulatory consultants and GMP auditors in the cosmetics industry
It applies to the entire product lifecycle, from raw material sourcing and production to packaging, storage, and distribution.
Whether you’re producing skincare, haircare, personal hygiene products, or decorative cosmetics, It ensures that your operations meet international safety and quality expectations.
Want to assess how this ISO Certification applies to your cosmetic facility? Contact us at support@pacificcert.com!
What is the ISO 22716 certification process?
ISO 22716 is widely used as a certification for cosmetic GMP compliance. Certification is voluntary, but it is often required by clients and retailers, especially for export into regulated markets such as the EU and Asia-Pacific.
Steps in the Certification Process:
- Gap Analysis – Compare current manufacturing practices with ISO 22716 requirements
- GMP System Development – Draft SOPs, quality manuals, and training materials
- Implementation – Introduce procedures across production, hygiene, personnel, documentation, and quality control
- Internal Audit – Evaluate compliance and prepare for third-party assessment
- Third-Party Audit – Conducted by an accredited certification body like Pacific Certifications
- Corrective Actions & Certification Issuance – Resolve any nonconformities and receive certification
We at Pacific Certifications guide cosmetic manufacturers through every step of this process. Contact support@pacificcert.com!
How to Implement ISO 22716 in Your Organization?
Implementation involves integrating GMP principles into your day-to-day cosmetic production and handling processes. Start by appointing a quality management representative and creating a GMP team that includes representatives from production, quality control, warehousing, and HR.
Develop and document standard operating procedures (SOPs) for:
- Personnel hygiene and training
- Equipment cleaning and calibration
- Raw material handling and storage
- Batch production and process controls
- Labelling and packaging
- Complaint handling and product recall
- Environmental control (air, water, lighting, ventilation)
Training, risk analysis, and regular audits are essential to ensure that practices align with both ISO 22716 and national cosmetic regulations.
Need help with auditing your GMP system? Email us at support@pacificcert.com!
Documentation Required
To demonstrate ISO 22716 compliance, the following documents are required:
- GMP policy and quality objectives
- SOPs for all critical production and storage activities
- Personnel hygiene and training records
- Batch manufacturing and packaging records
- Equipment cleaning and calibration logs
- Supplier qualification and raw material specifications
- Change control and deviation reports
- Complaint handling and product recall records
- Internal audit reports and management reviews
Eligibility Criteria
ISO 22716 is suitable for:
- Cosmetic manufacturing units (mass-market and boutique)
- Private label and contract manufacturing organizations (CMOs)
- Packaging and labeling firms in the cosmetics industry
- Raw material suppliers who handle and store cosmetic ingredients
- Exporters and importers operating in regulated markets
- Startups seeking compliance for product launches or EU notifications
Need a pre-certification eligibility review? Contact us at support@pacificcert.com!
Certification Timeline
Week | Activities |
Week 1 | Initial assessment, scoping, and GMP planning |
Week 2 | SOP development, hygiene controls, and documentation setup |
Week 3 | Staff training, implementation, and operational control review |
Week 4 | Internal audit and corrective actions |
Week 5 | Third-party audit and nonconformity resolution |
Week 6 | Final certification and GMP compliance endorsement |
Want to align your cosmetic operations with ISO 22716? Contact support@pacificcert.com.
ISO 22716 Certification Costs
The cost of ISO 22716 certification depends on:
- Size and complexity of your production facility
- Number of product categories and process lines
- Existing documentation and hygiene infrastructure
- Integration with other management systems (ISO 9001, ISO 14001)
- Training and internal audit needs
We offer customized pricing based on a site review and scope definition. Email support@pacificcert.com for a quote.
Clauses
Clause No. | Clause Title | Description |
1 | Scope | Defines the scope limited to cosmetic GMP guidelines. |
2 | Normative References | References to ISO standards essential for the application of ISO 22716. |
3 | Terms and Definitions | Provides terminology and definitions used throughout the document. |
4 | Personnel | Requirements for hygiene, qualifications, and responsibilities of staff. |
5 | Premises | Specifies the requirements for buildings and their suitability for operations. |
6 | Equipment | Guidance on the design, maintenance, and cleanliness of manufacturing equipment. |
7 | Raw Materials and Packaging Materials | Requirements for reception, storage, and handling of materials. |
8 | Production | Describes GMP for the manufacturing process to ensure quality and safety. |
9 | Finished Products | Guidelines for storage, shipment, and quality verification of final products. |
10 | Quality Control Laboratory | Requirements for testing, documentation, and control of quality parameters. |
11 | Treatment of Product that is Out of Specification | Procedures for handling non-conforming products. |
12 | Wastes | Management of waste to avoid contamination and ensure regulatory compliance. |
13 | Subcontracting | GMP responsibilities when outsourcing manufacturing or services. |
14 | Deviations | Managing and documenting deviations from established procedures. |
15 | Complaints and Recalls | Procedures for product complaints and product recall management. |
16 | Change Control | Requirements for managing changes that may affect product quality. |
17 | Internal Audit | Guidelines for conducting self-inspections and internal audits. |
18 | Documentation | Covers recordkeeping, SOPs, and traceability of GMP processes. |
Need help aligning your GMP practices with each clause? Contact support@pacificcert.com!
What are the requirements of ISO 22716?
To comply with ISO 22716, organizations must:

- Appoint qualified personnel and define GMP responsibilities
- Maintain clean, hygienic, and controlled premises
- Use validated, well-maintained equipment
- Establish SOPs for handling materials and conducting production
- Implement documentation and record-keeping systems
- Conduct regular internal audits and staff training
- Establish quality control testing and verification protocols
- Handle complaints and non-conforming products through formal procedures
- Ensure product traceability throughout the manufacturing and distribution process
- Implement change control and continual improvement processes
What are the benefits of ISO 22716?
Below are the key benefits of implementing ISO 22716:
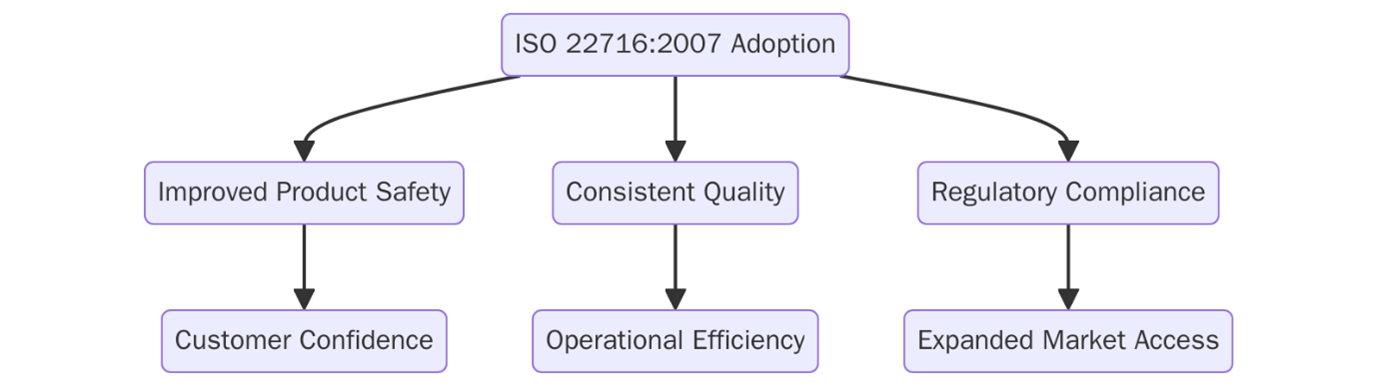
- Reduces contamination, mislabeling, and variability through controlled processes.
- Meets GMP requirements under EU Regulation 1223/2009, FDA standards, and ASEAN guidelines.
- Required for entering markets in Europe, Asia-Pacific, Latin America, and the Middle East.
- Structured documentation, training, and process controls lower the risk of batch failures and product returns.
- Strengthens supplier qualification, material traceability, and documentation across all stages.
- Certification demonstrates commitment to high-quality standards—crucial for retail partnerships and private-label clients.
- Can be integrated with ISO 9001 (quality), ISO 14001 (environment), and ISO 22717/18 (microbiological standards).
- Promotes lean manufacturing, efficient material use, and environmentally conscious production.
ISO 22716 is recognized globally as the GMP benchmark for cosmetics. With rising concerns over microbiological contamination and clean beauty standards, both regulators and consumers demand GMP-certified operations.
According to Statista, the global cosmetics market is expected to exceed USD 450 billion by 2027, and ISO 22716 certification is fast becoming a prerequisite for entering this competitive market, especially for private labels and e-commerce sellers.
Retailers such as Sephora, Boots, and Ulta increasingly require ISO 22716-compliant documentation from suppliers, while EU and ASEAN regulations demand strict GMP adherence for market entry.
Want to stay ahead in the global cosmetics market? Contact us to get certified for ISO 22716 at support@pacificcert.com!
How Pacific Certifications Can Help?
We offer end-to-end support for ISO 22716 implementation and certification:
- GMP gap analysis and documentation
- Audit support
- Staff training and awareness programs
- Internal audits and mock certification audits
- Certification issue
Whether you’re launching a new line or scaling operations, we help ensure compliance and certification success.
Ready to begin your ISO 22716 journey? Contact us at support@pacificcert.com!
FAQ on ISO 22716
Is ISO 22716 certifiable?
Yes, cosmetic companies can obtain third-party certification for compliance with ISO 22716.
Is ISO 22716 mandatory for cosmetics in the EU?
Yes, GMP compliance (based on ISO 22716) is mandatory under Regulation (EC) No 1223/2009.
Can it be used by small manufacturers or startups?
Yes, ISO 22716 is scalable and suitable for businesses of all sizes.
Does it apply to contract manufacturers?
Absolutely, CMOs and private labelers are often required to be GMP compliant.
Can ISO 22716 integrate with ISO 9001?
Yes, it complements ISO 9001 and other quality or safety management systems.
Ready to get ISO 22716 certified?
Contact Pacific Certifications to begin your certification journey today!
Suggested Certifications –
Read more: Pacific Blogs






