Introduction to ISO 14698-1:2003
ISO 14698-1:2003 is an essential international standard that addresses the control of biocontamination within cleanrooms and other associated controlled environments. While ISO 14644 primarily addresses particulate contamination, ISO 14698 focuses on microbiological contamination, ensuring cleanrooms meet rigorous biological safety and hygiene standards crucial for industries such as pharmaceuticals, biotechnology, healthcare, and food processing.

This standard specifies the principles and basic methodology for assessing and controlling biocontamination, providing a systematic framework to detect, monitor, and minimize microbial risks within clean environments.
Understanding the ISO 14698 Series
The ISO 14698 family is divided into key parts:
- ISO 14698-1:2003: General principles and methods for biocontamination control, risk assessment, and validation of microbiological control systems.
- ISO 14698-2:2003: Provides a set of guidelines for evaluating and selecting biocontamination control agents, such as disinfectants, and validating their performance in cleanroom settings.
Certification Process and Procedure
Step-by-Step Certification Journey:
- Initial Assessment and Risk Analysis:
- Comprehensive review of current microbiological control measures.
- Risk assessment to identify potential sources and pathways of microbial contamination.
- Documentation Development:
- Develop or refine biocontamination control plans.
- Define microbial monitoring strategies, including sample collection, incubation, and analysis protocols.
- Implementation Phase:
- Execution of contamination control measures.
- Staff training on aseptic techniques, gowning procedures, cleaning, and disinfection practices.
- Validation and Monitoring:
- Validate cleaning and disinfection processes.
- Establish routine environmental monitoring programs.
- Internal Audits and Corrective Actions:
- Conduct internal audits focusing on biological contamination risks.
- Implement corrective actions to mitigate identified risks.
- Management Review:
- Conduct regular reviews to evaluate the effectiveness of the biocontamination control system.
- Certification Audit:
- Engage an accredited certification body for external evaluation and certification based on ISO 14698-1 compliance.
- Certification Issuance:
- Achieve certification upon successful audit and compliance verification.
Documents Required for Certification
- Biocontamination Control Manual
- Environmental Monitoring Plans and Records
- Microbial Risk Assessment Reports
- Cleaning and Disinfection Procedures
- Validation Reports for Biocontamination Control Measures
- Staff Training and Competency Records
- Internal Audit Reports and Corrective Action Plans
- Management Review Records
Eligibility Criteria
Organizations involved in industries that operate cleanrooms and require stringent microbial control are eligible, including:
- Pharmaceutical manufacturers
- Biotechnology laboratories
- Hospitals and healthcare facilities
- Medical device manufacturers
- Food and beverage industries
- Cosmetic product manufacturers
Certification Fees
Certification costs vary depending on organizational size, cleanroom complexity, and operational risk factors. For a detailed quotation tailored to your needs, contact Pacific Certifications at support@pacificcert.com.
Certification Duration
The overall timeline for certification typically includes:
- Preparation and planning: 3-5 months
- Implementation and validation: 2-4 months
- Internal audit and corrective actions: 1-2 months
- Certification audit and issuance: 1 month
Total duration usually spans between 7-12 months, contact us at support@pacificcert.com to know more!
Requirements of ISO 14698-1 Certification
To achieve ISO 14698-1 certification, organizations must implement a comprehensive framework focused on identifying, monitoring, controlling, and mitigating microbial contamination risks in cleanrooms and controlled environments. The requirements cover risk assessment, environmental monitoring, personnel training, and validation of contamination control measures:
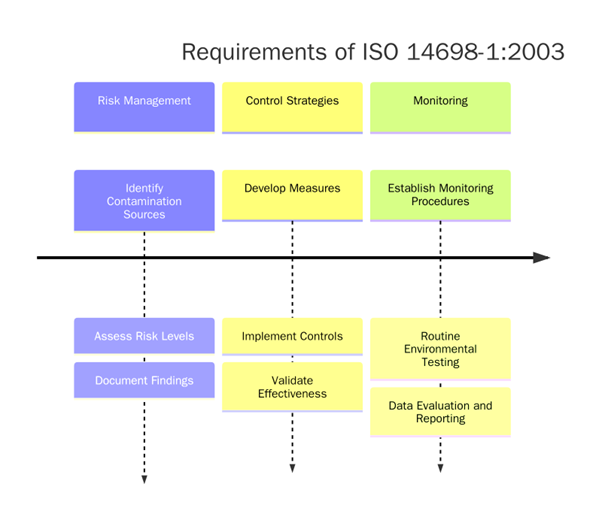
- Microbial Risk Assessment: Identify and evaluate potential contamination sources.
- Environmental Monitoring Program: Implement microbiological monitoring, including air, surface, and personnel sampling.
- Contamination Control Strategy: Develop and enforce rigorous cleaning and disinfection protocols.
- Personnel Training: Regular training on aseptic techniques, hygiene practices, and gowning procedures.
- Validation of Control Measures: Validate cleaning agents, disinfectants, and air filtration systems.
- Corrective and Preventive Actions: Prompt action plans for contamination incidents and process deviations.
- Data Analysis and Trending: Analyze microbial data trends for proactive contamination control.
- Management Involvement: Active management participation in system reviews and improvement initiatives.
Benefits of ISO 14698-1 Certification
Achieving ISO 14698-1 certification brings numerous advantages to organizations operating cleanrooms and controlled environments. Certification ensures better microbial control and enhances regulatory compliance and customer confidence, positioning organizations strongly in competitive markets. Below are the key benefits:

- Microbial Safety: Ensures minimal microbial contamination risks.
- Compliance: Meets global health and safety requirements (e.g., FDA, EU GMP, WHO).
- Customer Confidence: Boosts market reputation for producing safe, high-quality products.
- Contamination Events: Lowers risk of costly product recalls, batch failures, and regulatory penalties.
- Excellence: Streamlines cleanroom operations through validated control measures.
- Market Access: Facilitates entry into strict-regulation markets requiring documented biocontamination control systems.
- Staff Competency: Elevates personnel performance and awareness of microbiological hazards.
- Continuous Improvement: Promotes ongoing review, adaptation, and strengthening of microbial control practices.
With stricter health and safety regulations globally, organizations are prioritizing biocontamination control more than ever. The COVID-19 pandemic has further accelerated the demand for validated cleanroom operations and microbial risk mitigation, making ISO 14698-1 compliance a strategic necessity for companies in pharmaceutical, healthcare, and high-tech manufacturing sectors.
How Pacific Certifications Can Help
Pacific Certifications offers end-to-end support for ISO 14698-1 certification:
- Specialists in microbiological risk assessment and cleanroom certification.
- Certification accredited by ABIS ensures worldwide acceptance.
- Customized support throughout risk assessment, monitoring setup, and system validation.
- Ongoing surveillance audits and continuous improvement support.
Start your ISO 14698-1 certification journey today by contacting Pacific Certifications at support@pacificcert.com.
Frequently Asked Questions (FAQs)
While not mandatory by law, certification demonstrates commitment to microbial safety and regulatory compliance, often required by clients and regulatory agencies.
Through environmental monitoring programs that include air sampling, surface swabbing, and personnel monitoring using standardized microbiological methods.
Yes, organizations often integrate ISO 14698-1 with ISO 14644-1 for comprehensive particulate and microbiological contamination control.
Monitoring frequency depends on cleanroom classification, operational risk, and historical contamination trends but is typically performed daily or per shift in critical areas.
Immediate investigation, corrective actions, and preventive measures must be initiated, and a thorough root cause analysis should be performed.
Ready to get ISO 14698-1:2003 certified?
Contact Pacific Certifications to begin your certification journey today!
Suggested Certifications –








