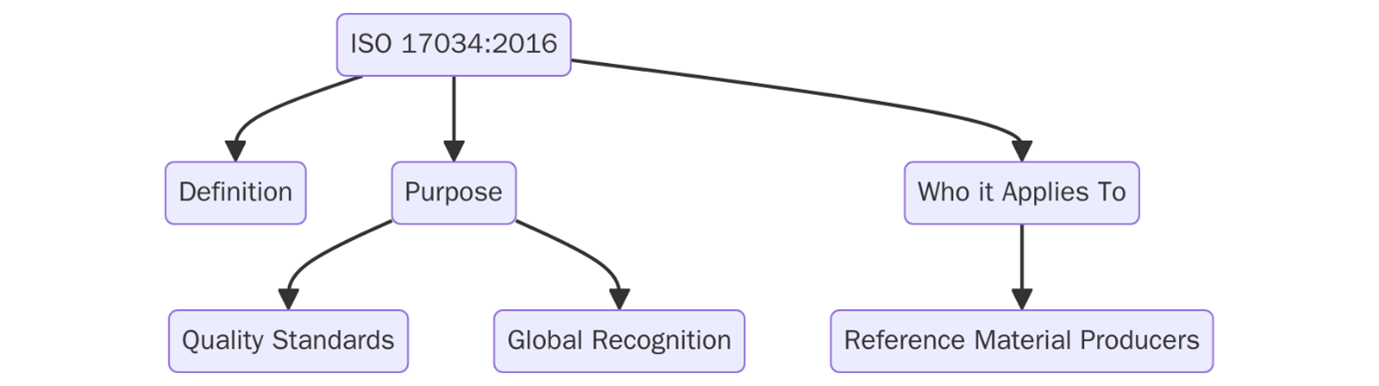
What is ISO 17034?

ISO 17034:2016, specifies the general requirements for competency of reference material producers. Reference materials (RMs) are described as substance or materials with well-defined property values that are used for the calibration of measuring instruments, validation of measurement methods, or assessment of measurement performance. The aim of ISO 17034:2016 is to make certain that reference materials are consistently produced to a known quality and traceability. This is applicable to many industries such as pharmaceuticals, environmental testing, and manufacturing.
Obtaining ISO 17034:2016 certification shows that producers have the capability to produce high quality reference materials that are reliable and traceable to international standards, which is vital for accurate measurement when testing and calibrating various applications.
For ISO 17034 certification assistance please reach out to us at support@pacificcert.com.
Purpose
ISO 17034 provides a standard for reference material producers and producers that want to produce fit-for-purpose reference material. The overall purpose is to provide a standardized and consistent framework for reference material producers to ensure the quality and reliability of the reference material produced. The standard focuses on competence in the form of organizational structure, management system, and production processes to ensure that the reference materials it produces are fit-for-purpose and in compliance with international measurement standards. It is also the aim of ISO 17034:2016 to establish a worldwide benchmark for the production of reference material in order to ensure reference materials can be relied upon for calibration, quality assurance and testing purposes.
Scope and Applicability
ISO 17034:2016 applies to any organization involved in the production of reference materials. It is particularly relevant to industries where precision and measurement integrity are essential, such as pharmaceuticals, environmental testing, oil and gas, food safety, and manufacturing. The standard sets the framework for producing materials that can be reliably used for calibration, validation, and quality assurance in a variety of scientific and industrial applications. Organizations from these sectors must adhere to this standard to ensure their reference materials meet internationally recognized quality and traceability requirements, ultimately ensuring the accuracy of measurement instruments and testing systems across different applications.
This standard helps organizations produce reference materials that meet high quality, safety, and regulatory standards across these and other industries.
Key Definitions
- Reference material (RM): A material with a known or certified property, primarily for the purposes of calibration, validation, or quality assurance in relation to testing.
- Traceability: The property of a set of measurement results that can be traced back to a national or international standard and is linked by an unbroken chain of calibrations.
- Certification: The process by which an independent organization/ body verifies that reference materials comply with the specifications for the relevant ISO 17034:2016 standard.
Clause-wise Structure of ISO 17034
Clause Number | Title | Description |
Clause 1 | Scope | Defines the scope of the standard and its applicability to reference material producers. |
Clause 2 | Normative References | Lists the related standards that must be followed alongside ISO 17034:2016. |
Clause 3 | Terms and Definitions | Provides essential definitions used in the standard. |
Clause 4 | General Requirements | Outlines the need for a management system to ensure consistent quality. |
Clause 5 | Structural Requirements | Specifies the need for a robust organizational structure to support quality production. |
Clause 6 | Competence Requirements | Describes the qualifications and competencies needed by personnel involved in the production of reference materials. |
Clause 7 | Production Requirements | Details the required processes for producing and characterizing reference materials. |
Clause 8 | Management System Requirements | Requires the implementation of quality management systems to support ongoing compliance. |
What are the requirements of ISO 17034?
ISO 17034 establishes several important requirements for reference material producers. First, a Quality Management System (QMS) must be established to ensure that the reference materials are of required quality and that processes are controlled and consistent. Below are the other requirements:
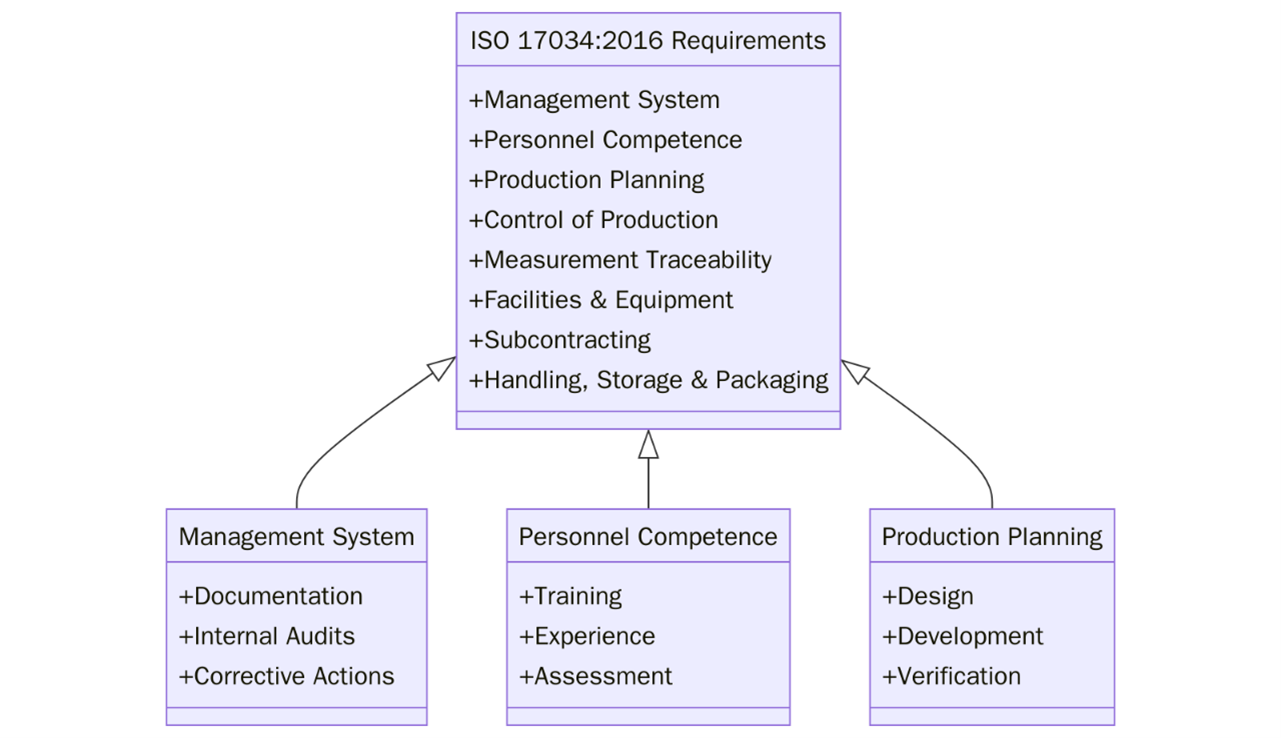
- The organization must be legally identifiable, operate impartially, ensure confidentiality, and have adequate liability coverage.
- A defined organizational structure and a documented quality management system must be in place, with appointed competent personnel responsible for quality and technical functions.
- Adequate resources must be maintained, including qualified personnel, suitable facilities with controlled environments, and calibrated and maintained equipment.
- Subcontracted activities must be performed by competent providers under controlled conditions.
- A documented production plan must govern all steps of reference material (RM) development, selection, preparation, characterization, homogeneity, stability, packaging, labeling, and distribution.
- Measurement procedures must be validated, results must be traceable to SI units or recognized references, and uncertainties must be properly evaluated.
- RMs must be uniquely identified, handled under controlled conditions, and stored/transported to maintain integrity and traceability.
- The system must include controls for document management, internal audits, handling of complaints/nonconformities, and actions for continual improvement.
For more information on the certification process, contact our team at support@pacificcert.com.
What are the benefits of ISO 17034?
ISO 17034 helps reference material producers to demonstrate technical competence and produce high-quality, reliable reference materials (RMs) and certified reference materials (CRMs). Below are the key requirements:
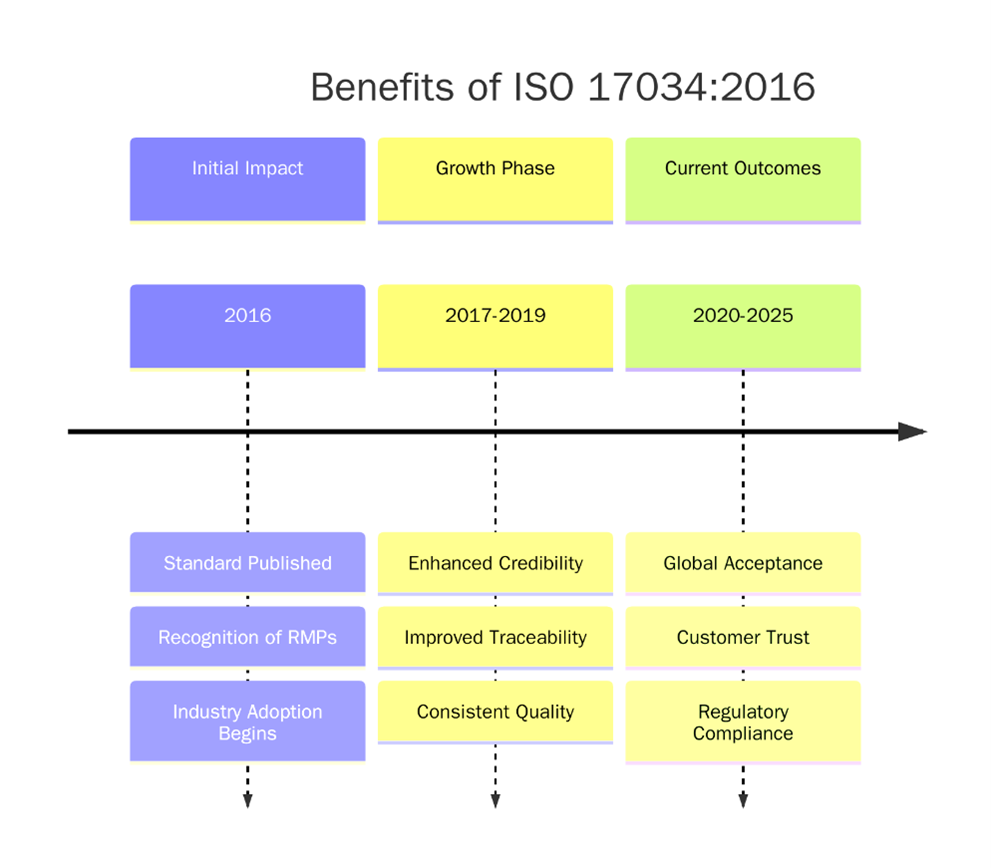
- Demonstrates technical competence in producing high-quality reference materials.
- Enhances credibility and global recognition among laboratories and regulatory bodies.
- Ensures metrological traceability of measurement values to international standards.
- Improves confidence in test results used for calibration, validation, and quality control.
- Supports regulatory and accreditation compliance across various industries.
- Reduces risks of nonconformities by ensuring consistent production processes.
- Facilitates acceptance of RMs/CRMs internationally, boosting market reach.
- Promotes continual improvement in production and quality management systems.
Major industry players including Merck, Agilent, Thermo Fisher, LGC, and Waters have expanded their ISO 17034-accredited capabilities, reflecting the growing importance of standard-compliant materials for regulatory submissions. Additionally, the scope of reference materials has diversified to include emerging domains such as nanomaterials, cannabis testing, and optical spectroscopy, further expanding the applicability of ISO 17034. On a broader scale, ISO 17034 plays an important role in supporting international trade and laboratory accreditation.
Contact us today at support@pacificcert.com to get your process started!
Certification Process
The certification process includes the following steps:
- Pre-Certification Assessment: which will assist in determining the gaps in your operation.
- The Documentation Review: to review and ensure that existing processes and systems meet your standard.
- Stage 1 Audit: to audit to evaluate that you meet the requirements of a Management System.
- Stage 2 Audit: which is a full on-site audit to evaluate your processes, people, and practices of production.
- Certification Decision: which is a decision the certification body will make determined by their audit findings in order to grant you certification.
- Ongoing Monitoring: as the certification body will have a requirement that ongoing monitoring needs to occur to ensure continued compliance.
Timeline for ISO 17034 Certification?
The timeline for ISO 17034:2016 certification spans several months. The pre-assessment and preparation phase may take 1–2 months, during which the organization reviews and adjusts its practices to meet ISO 17034 requirements. After this, certification issuance generally occurs within 3–6 months, depending on audit outcomes and the organization’s readiness.
What is the Cost ?
The cost of ISO 17034 certification can vary depending on several factors, including the size of the organization, complexity of operations, and the number of reference materials produced. Generally, the cost will include:
- Audit Fees: Fees for external audits by certification bodies.
- Training and Implementation Costs: Expenses for training personnel and updating processes.
- Ongoing Maintenance: Costs for internal audits and recertification every three years.
For a personalized quote, contact support@pacificcert.com.
How Pacific Certifications Can Help?
At Pacific Certifications, we specialize in providing ISO 17034:2016 certification services. Our experienced auditors guide you through the entire certification process, from pre-assessment to certification. We focus on helping your organization implement robust quality management systems and ensure ongoing compliance with the ISO standards.
Our Role Includes:
- Stage 1 and Stage 2 audits to evaluate documentation and technical procedures.
- Objective conformity assessments based on impartial auditing principles aligned with ISO/IEC 17021-1.
- Certification issuance upon successful completion of audit
- Ongoing surveillance audits to maintain compliance
- Support for multi-site or global operations
- Rapid processing and efficient audit scheduling
- Recognition across regulatory and accreditation bodies, enabling your CRMs to be accepted globally.
For more information or to initiate the certification process, please contact us at support@pacificcert.com.
ISO 17034 Training and Courses
ISO 17034:2016 trainings are designed to equip individuals and teams with a deep understanding of the structure and how to implement or audit these systems effectively within an organization. There are several levels of training available:
- Lead Auditor Training – Equips professionals to conduct external third-party audits.
- Lead Implementer Training – For those responsible for planning and executing ISO 17034 implementation.
- Internal Auditor Training – Preparing internal auditors for certification audits.
Pacific Certifications provides accredited training programs. If your organization is looking for ISO training, our team is equipped to help you, contact us at support@pacificcert.com
FAQ: ISO 17034
How long does it take to get ISO 17034 certification?
The certification process typically takes 3–6 months, depending on your organization’s preparedness and audit outcomes.
Is ISO 17034 certification mandatory for all industries?
While it is not legally required, ISO 17034 certification is essential for organizations in industries where precision in calibration and testing is critical.
Can I apply for ISO 17034 certification if I do not have an established quality management system?
No, ISO 17034 requires an established quality management system to ensure that the reference materials produced meet high-quality standards.
What are the costs involved in ISO 17034 certification?
Costs vary depending on the size and complexity of the organization. It includes audit fees, training, and process improvements.
How often do I need to renew ISO 17034 certification?
ISO 17034 certification is valid for three years, after which a recertification audit is required.
Contact Us
If you need support with ISO 17034:2016 certification, contact us at support@pacificcert.com.
Read More at: Blogs by Pacific Certifications






