In the highly regulated medical device industry, ensuring that products remain sterile until point-of-use is critical. ISO 11607-1:2019 sets the international benchmark for packaging systems that maintain sterility and protect the integrity of terminally sterilized medical devices.
Looking to obtain ISO 11607-1:2019 certification? Reach out to us today at support@pacificcert.com, call us at +91-8595603096, or visit www.pacificcert.com to learn more about our globally recognized certification services.
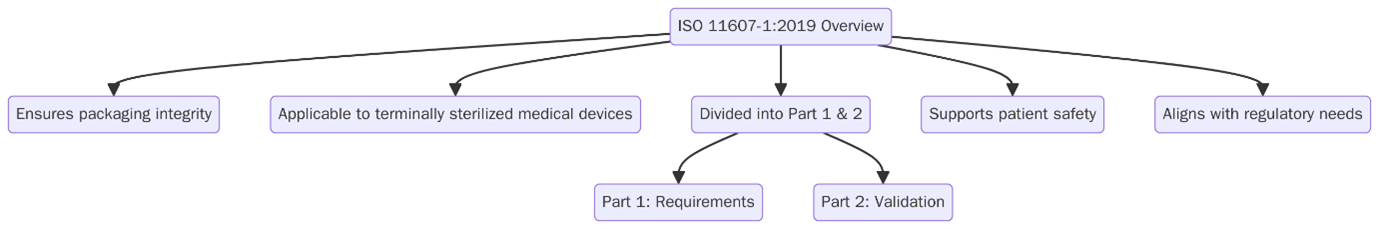
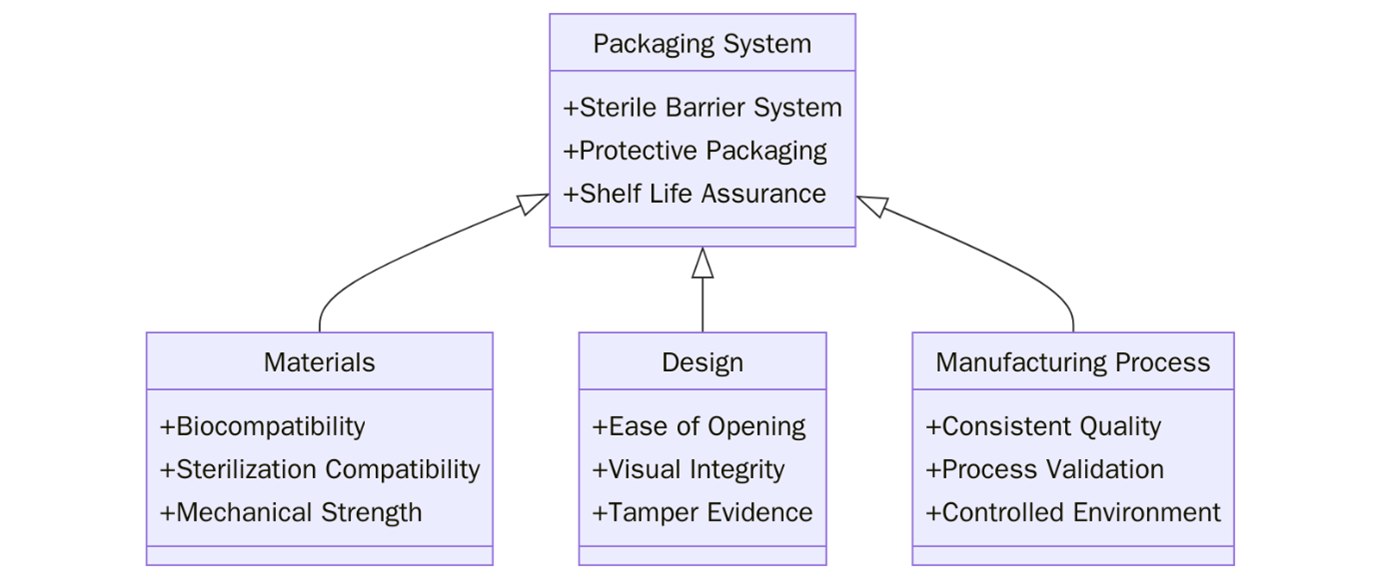
ISO 11607-1:2019 defines the requirements and test methods for materials, sterile barrier systems, and packaging systems used for terminally sterilized medical devices. This standard is essential for ensuring that a medical device, once sterilized, maintains its sterility during transport, storage, and until the point of use.
The standard applies to healthcare manufacturers and suppliers who are involved in the design, production, and distribution of packaging systems intended for terminal sterilization.
ISO 11607-1:2019 is closely aligned with ISO 13485 (Quality Management Systems for Medical Devices) and is frequently referenced by regulatory bodies such as the FDA and the European Medical Device Regulation (MDR).
Get certified with confidence! If you’re aiming for ISO 11607-1:2019 certification, our experts are here to help. Contact us at support@pacificcert.com, call +91-8595603096, or explore our services at www.pacificcert.com.
To comply with ISO 11607-1:2019, organizations must meet specific packaging design, performance, and validation requirements:
To start your certification process, simply contact our team at support@pacificcert.com, call us at +91-8595603096, or visit our official website at www.pacificcert.com. We provide complete support from documentation to final certification.
The global medical device packaging market is projected to reach USD 50 billion by 2028, driven by increasing demand for safe, sterile products. Stringent regulatory scrutiny and growing emphasis on patient safety are prompting manufacturers to adopt robust packaging standards like ISO 11607-1. There is also a growing trend toward sustainability in healthcare packaging, encouraging the development of environmentally friendly materials that still meet sterilization and barrier requirements. Moreover, digital traceability and smart packaging technologies are being integrated into medical device packaging to enhance monitoring, compliance, and lifecycle management. These evolving dynamics make ISO 11607-1 certification more critical than ever.
Thank you for your interest in ISO 11607-1:2019 certification. For detailed information, pricing, and documentation requirements, feel free to email us at support@pacificcert.com, call us directly at +91-8595603096, or check out www.pacificcert.com.
Any organization involved in the packaging of terminally sterilized medical devices can benefit significantly from ISO 11607-1 certification.
For ISO 11607-1:2019 certification inquiries,
Email: support@pacificcert.com, Phone: +91-8595603096 &Website: www.pacificcert.com
Your compliance is our commitment!
ISO 11607-2:2019 is the second part of the ISO 11607 series, focusing specifically on the validation of forming, sealing, and assembly processes used to create sterile barrier systems for medical devices. While ISO 11607-1 addresses the materials and design requirements for sterile packaging, ISO 11607-2 ensures that the manufacturing processes involved are consistently capable of producing compliant packaging. This includes the qualification of equipment, process validation, and ongoing process control to maintain package integrity. It is essential for manufacturers who perform final packaging operations, as it provides assurance that their packaging processes meet rigorous quality standards and are reproducible under routine production conditions.
At Pacific Certifications, we provide expert audit and certification services for ISO 11607-1 and ISO 11607-2. As an accredited certification body, we ensure an impartial and thorough evaluation of your packaging systems against the latest international standards.
Our Certification Services Include:
Our experienced auditors bring deep industry knowledge, helping clients achieve certification and enhance their quality and regulatory systems.
Pacific Certifications is accredited by ABIS, in case you need support with ISO 11607-1:2019 certification for your business, please contact us at suppport@pacificcert.com or +91-8595603096
It applies to the packaging of medical devices that are sterilized by the manufacturer and are intended to remain sterile until opened for use.
While not mandatory, ISO 11607-1 is widely accepted by the FDA as an effective method to demonstrate compliance with sterile packaging requirements.
Part 1 deals with the design and performance requirements, while Part 2 focuses on validation requirements for forming, sealing, and assembly processes.
Typically, ISO 11607-1 certification is valid for three years with annual surveillance audits.
As a certification body, we do not provide consultancy or implementation services, but we can guide you through the certification process and clarify requirements.
Contact Pacific Certifications to begin your certification journey today!
Suggested Certifications –

Get in touch!
This will close in 0 seconds
Get in touch!
This will close in 0 seconds
WhatsApp us