What is EN 14683:2019?

EN 14683:2019 is a European standard that specifies construction and performance requirements for medical face masks used in healthcare settings. These masks serve as a barrier to minimize the transmission of infectious agents from healthcare professionals to patients and vice versa. The standard defines mask types, bacterial filtration levels, breathability, and splash resistance to guide manufacturers and hospitals in choosing safe and tested face protection.
In hospitals, clinics, and dental environments, EN 14683-certified masks help limit the risk of contamination during procedures where respiratory droplets may be produced. The standard is frequently referenced during procurement of protective equipment, especially in infection-prone settings such as operating theatres and isolation wards.
To begin your ISO journey or schedule an audit contact support@pacificcert.com
Purpose
The main objective of EN 14683:2019 is to establish performance levels and safety benchmarks for medical face masks. It fills the need for verified filtration capability and consistent product quality. With growing awareness of airborne pathogens, this standard helps organizations reduce exposure risks by relying on scientifically validated barrier properties.
Scope and Applicability
EN 14683 applies to medical face masks intended to limit the transmission of infectious agents. These masks are worn by healthcare personnel in operating rooms, intensive care units, outpatient clinics, and other clinical areas. The standard also applies to settings outside hospitals where infection control is necessary such as elder care homes, dental practices, diagnostic labs, and emergency services.
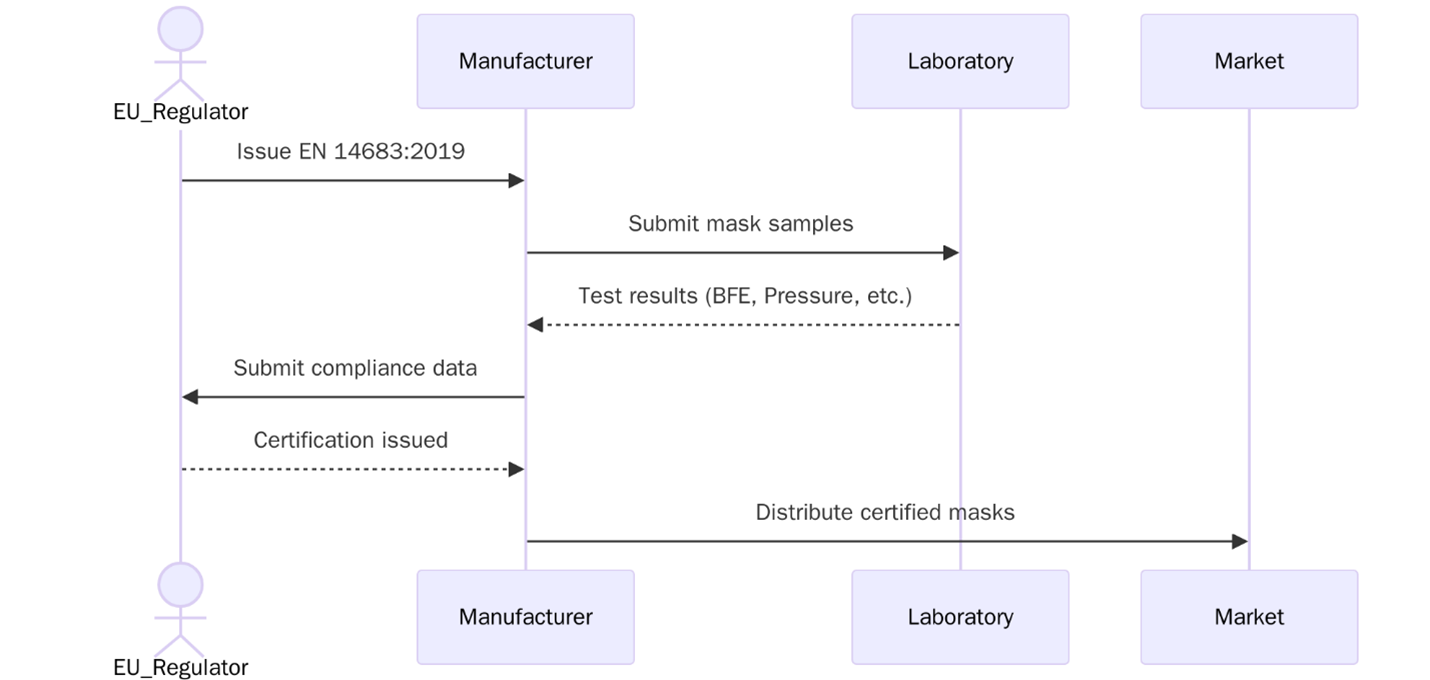
It is relevant to manufacturers, importers, and healthcare providers responsible for the selection and supply of personal protective equipment.
Key Definitions
- Medical face mask: A mask that covers the mouth and nose to serve as a barrier against infectious materials
- Bacterial Filtration Efficiency (BFE): The ability of a mask to filter bacteria-containing aerosol
- Differential pressure (Pa/cm²): A measurement of breathability, indicating air permeability of the mask
- Splash resistance pressure: The mask’s ability to resist penetration by synthetic blood under pressure
- Microbial cleanliness: The number of viable microorganisms on the mask before use
Structure of EN 14683:2019
Number | Title | Description |
1 | Scope | Defines intended use and types of masks covered |
2 | Normative References | Lists referenced standards including testing protocols |
3 | Terms and Definitions | Provides definitions of technical and performance-related terms |
4 | Requirements | Describes performance criteria for BFE, breathability, splash resistance |
5 | Test Methods | Details laboratory testing approaches for evaluating mask characteristics |
6 | Classification | Explains mask types (Type I, II, IIR) based on performance levels |
7 | Marking and Labelling | Specifies how masks must be labelled with type, lot number, and BFE value |
8 | Manufacturer’s Instructions | Lists user instructions and storage guidance requirements |
Types of Medical Masks under EN 14683:2019
Type | BFE (%) | Differential Pressure (Pa/cm²) | Splash Resistance (mmHg) | Microbial Cleanliness (cfu/g) |
Type I | ≥ 95 | < 40 | Not required | ≤ 30 |
Type II | ≥ 98 | < 40 | Not required | ≤ 30 |
Type IIR | ≥ 98 | < 60 | ≥ 120 | ≤ 30 |
What are the requirements of EN 14683:2019?
EN 14683:2019 specifies several performance-based requirements for medical face masks to ensure adequate protection for both patients and healthcare professionals. The key requirement is Bacterial Filtration Efficiency (BFE), which assesses the mask’s ability to prevent bacteria-containing droplets from penetrating the material. Below are other requirements:
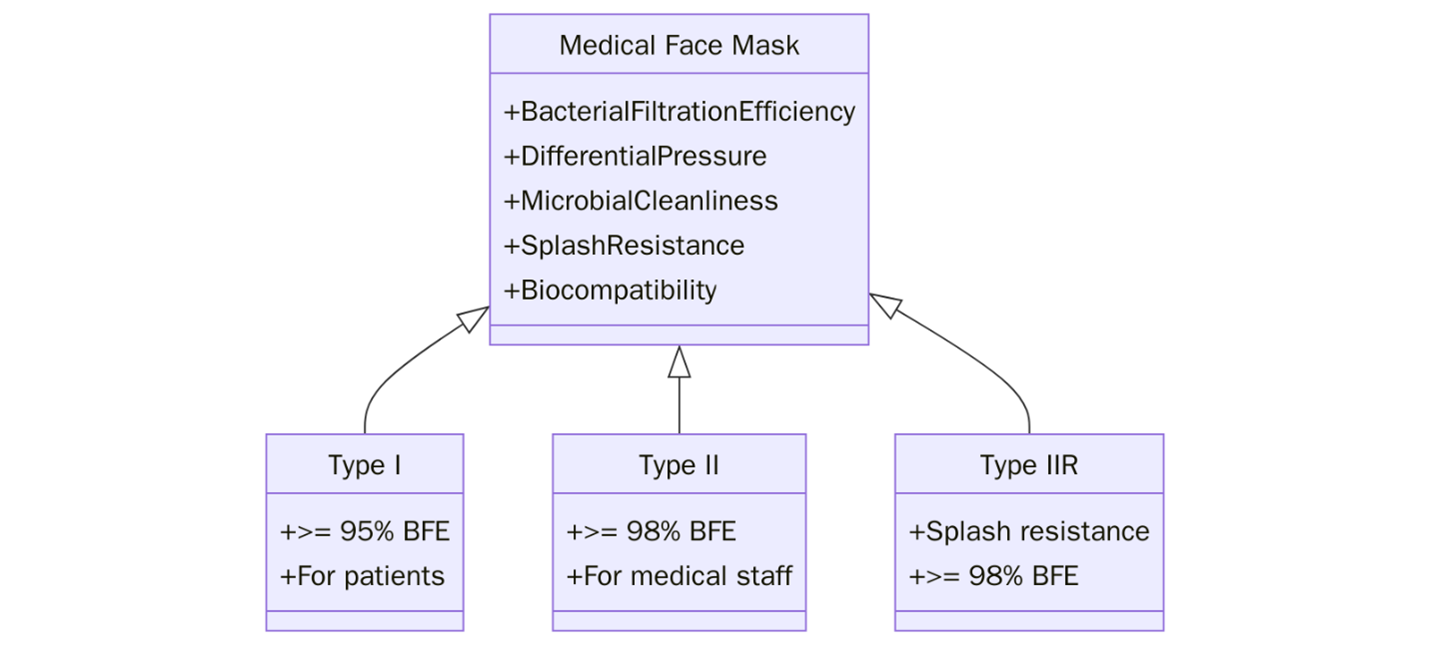
- Face masks must meet defined bacterial filtration efficiency thresholds, with Type I ≥ 95% and Type II/IIR ≥ 98%.
- Masks must pass differential pressure tests to ensure breathability without discomfort.
- Type IIR masks must show resistance to synthetic blood splash under set pressure conditions.
- Products must undergo microbial cleanliness assessment prior to packaging.
- All test results must be validated in accordance with referenced European standards.
- Masks must be designed for single use unless specifically tested and labeled otherwise.
- Each product must include full labeling with type, batch number, and performance rating.
- Packaging must include clear usage and disposal instructions to avoid contamination risk.
What are the benefits of EN 14683:2019 Certification?
EN 14683 certification supports product assurance and public health safety. It helps users confirm that protective masks perform as expected in real-world settings where respiratory infection risk is high. Below are key benefits.
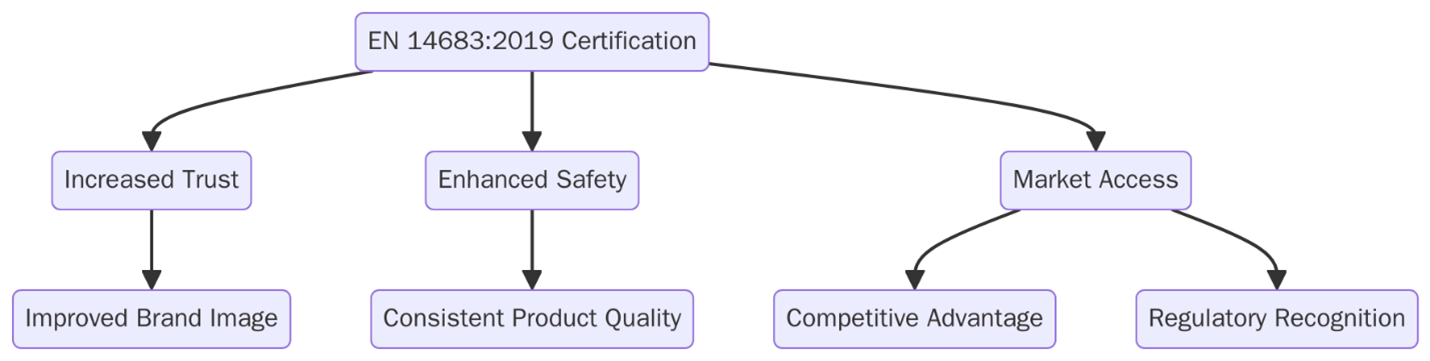
- Verified bacterial filtration confirms that masks meet minimum safety standards during patient care and aerosol-generating tasks.
- Tested breathability performance helps prevent discomfort for users who wear masks for long durations especially in surgery or intensive care.
- Reliable splash resistance for Type IIR masks reduces the chance of blood or fluid entry during invasive procedures.
- Increased product trust among procurement teams due to clearly labeled filtration and testing compliance data.
- Lower infection risk for both staff and patients as masks serve as an effective barrier against droplet spread.
- Better procurement decisions through defined classification and documented test results for performance comparison.
- Supports risk-based selection based on type of procedure or exposure level such as choosing IIR for high splash conditions.
- Enables global supply compatibility by referencing a harmonized standard accepted in the EU and many international markets.
Eligibility Criteria
EN 14683 certification can be applied for by any manufacturer or distributor of medical face masks. Organizations must have the technical capacity to test their products or work with an accredited lab. Firms already certified to ISO 9001 or ISO 13485 often have systems in place that support faster audit readiness due to existing quality documentation and traceability controls.
Certification Process: EN 14683:2019
Review
Initial review of product type, testing methods, and current quality records
Preparation
Manufacturer prepares documents including test data, design inputs, and packaging labels
- Document review to confirm readiness for performance validation and packaging compliance
- On-site or remote audit to examine processes, testing records, microbial controls, and traceability
- Final review and decision based on audit findings and performance criteria
- Yearly audits to check ongoing product conformity and record keeping
Timeline for EN 14683:2019 Certification
The entire certification process generally takes six to eight weeks for organizations that already have test data and documented quality systems. If testing must still be arranged, or additional design records are needed, the process may take longer. Timeframes also depend on the number of product variants and production sites under evaluation.
What is the cost of EN 14683:2019?
Costs vary depending on factors such as number of mask types, scope of the audit, whether testing is outsourced, and the quality documentation already available. Firms that integrate EN 14683 with ISO 13485 or ISO 9001 may benefit from shared audits. However, new manufacturers without any existing system should expect longer preparation and testing cycles that may influence pricing.
How can Pacific Certifications Help?
Pacific Certifications audits organizations for EN 14683:2019 to verify that medical face masks meet performance and documentation requirements. Our auditors review test data packaging controls labeling and microbial safety records to confirm full alignment with the standard.
We are accredited by ABIS and perform both standalone and combined audits with ISO 9001 or ISO 13485. Our team has experience working with medical device manufacturers including start-ups and high-volume producers.
Training and Courses
Lead Auditor Training
Covers how to assess conformity of face masks to EN 14683 including test records and labeling
Lead Implementer Training
Focuses on setting up internal processes for documentation, product validation, and packaging
Internal Auditor Training
Trains in-house staff to review ongoing testing, microbial controls, and classification of products
Pacific Certifications provides accredited training programs. Contact support@pacificcert.com to schedule your training.
FAQs
How long is the EN 14683 certification valid?
The certificate is valid for three years with annual surveillance audits.
Is EN 14683 applicable only to hospitals?
No, it is also used by dental clinics, diagnostic labs, and home care providers.
Can EN 14683 be audited together with ISO 13485?
Yes, many manufacturers combine these for streamlined audits.
Do masks need to be tested before audit?
Yes, testing by a recognized lab is necessary for certification.
What types of masks fall under this standard?
Type I, Type II, and Type IIR disposable medical face masks.
Is EN 14683 recognized outside Europe?
Yes, it is accepted in many countries as an equivalent safety benchmark.
Do small manufacturers need certification?
Yes, especially when supplying to hospitals or government agencies.
Ready to get ISO 14683:2019 certified?
Contact Pacific Certifications to begin your certification journey today!
Suggested Certifications –
Read more: Pacific Blogs






