What is CE Marking?

CE Marking is a self-declaration symbol placed on products to indicate conformity with European Union directives and regulations. It acts as a passport for goods sold within the European Economic Area (EEA). CE covers a wide range of product groups, including electronics to personal protective equipment. The mark confirms that a product meets all applicable safety, health, and environmental requirements.
From manufacturers to distributors rely on CE Marking to show their products meet legal access conditions in the EU. Products bearing the CE mark can move freely across member states without additional national approvals.
To begin your CE Marking process or request an audit, contact us at support@pacificcert.com
Purpose
The main objective of CE Marking is to ensure that products placed on the EU market are safe for use and do not harm users, the environment, or other property. It provides a unified system of conformity that removes trade barriers across Europe and brings consistency in product evaluation and labeling.
Scope and Applicability
CE Marking applies to over 20 different product categories. Some common examples include:
- Electrical and electronic equipment like computers, phones, and televisions
- Medical devices ranging from surgical instruments to diagnostic equipment
- Construction materials such as cement, structural steel, and fire safety components
- Machinery and industrial tools used in factories or workshops
- Toys, childcare items, and personal protective gear including masks and helmets
Products manufactured outside the EU but intended for sale within it must comply with CE requirements. The responsibility lies with the manufacturer or importer to assess the applicable directives and apply the correct procedures before labeling.
Key Definitions
Conformity Assessment: The process of checking whether a product meets relevant directives through testing, documentation, and internal controls.
EU Declaration of Conformity (DoC): A written statement confirming that a product meets the requirements of applicable EU legislation.
Notified Body: An external organization designated by an EU member state to conduct conformity assessments where required.
Technical Documentation: Records such as design files, risk assessments, and test reports that support the CE Marking claim.
Structure of CE Marking Regulations
| Section | Title | Description |
| 1 | Scope | Outlines categories and products subject to CE Marking |
| 2 | Definitions | Explains terms like harmonized standards and conformity assessment |
| 3 | Conformity Assessment | Sets out steps for verifying product conformity |
| 4 | Declaration of Conformity | Lists required content and retention of the DoC |
| 5 | Use of CE Mark | Describes size, placement, and conditions for applying the mark |
| 6 | Role of Notified Bodies | Defines when and how third-party assessments are required |
| 7 | Market Surveillance | Details authority checks and consequences for non-conforming products |
| 8 | Harmonized Standards | Links European standards to specific directive requirements |
What are the CE Marking requirements?
To apply CE Marking, companies must complete a set of steps based on the directives that apply to their product. Below are the key CE Marking requirements:
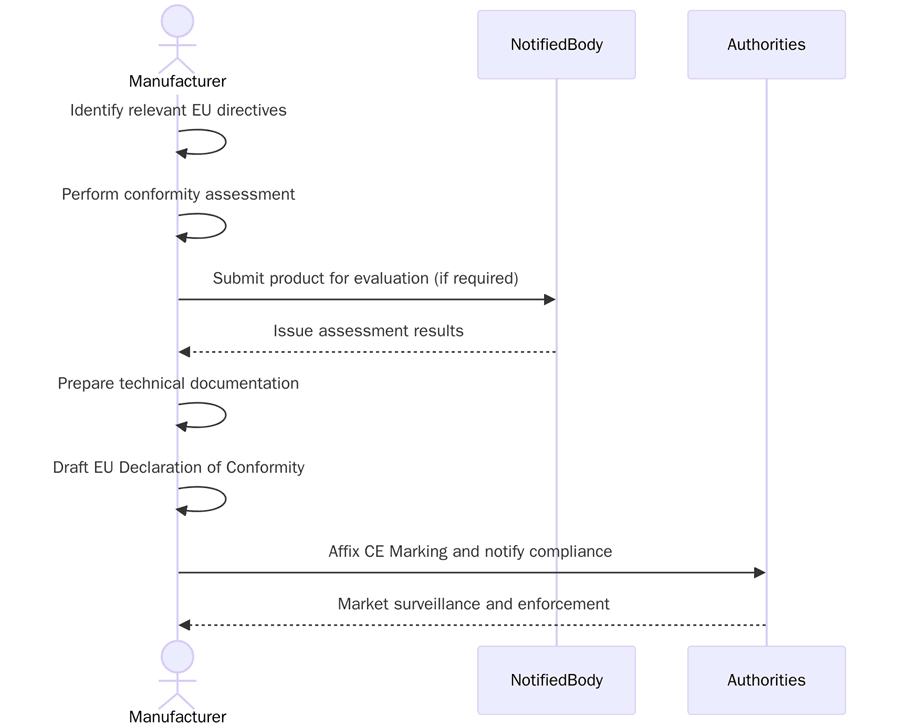
- Identify which EU directives apply to the product
- Review harmonized standards that support the directive
- Conduct product testing and risk analysis
- Compile technical documentation including design files and test data
- Prepare the Declaration of Conformity (DoC)
- Affix the CE Mark on the product and packaging
- Involve a Notified Body if required by the directive
- Maintain records for 10 years from the date of market placement
What are the benefits of CE Marking?
CE Marking supports legal market entry while also improving how a product is received by customers and regulators. It ensures that internal quality controls are in place and that claims can be supported if inspected.

- Allows unrestricted trade within the EU market
- Boosts trust in product safety and quality
- Reduces delays at customs or border inspections
- Supports third-party certifications like RoHS or MDR
- Avoids market withdrawals and legal penalties
- Helps establish credibility with procurement buyers
- May ease product acceptance in non-EU regions
CE enforcement has increased across Europe with tighter checks on technical files and importer responsibilities. Authorities now demand precise documentation and traceability from suppliers. Recent shifts in CE-related directives include new cybersecurity requirements under the Radio Equipment Directive and additional safety layers under the updated Machinery Regulation. Globally, CE is being used as a model in markets like Turkey and parts of the Middle East, expanding its influence beyond Europe.
Eligibility Criteria
CE Marking is mandatory for all manufacturers, importers, and authorized representatives placing products into the European market under applicable CE directives. Companies must maintain control over design, manufacturing, and material sourcing. For some product groups, third-party assessment from a Notified Body is required. Firms certified to ISO 9001 or ISO 13485 often find that their internal systems help meet CE requirements more smoothly.
CE Marking Certification Process
- Directive Identification – Review applicable CE directives and product categories
- Assessment Method Selection – Choose the conformity route (self-assessment or via Notified Body)
- Product Evaluation – Conduct design verification, safety testing, and risk reviews
- Technical File Creation – Compile required documents including risk assessments and test results
- Drafting the DoC – Prepare the Declaration of Conformity referencing applicable standards
- Affixing the CE Mark – Apply the CE mark visibly on the product, packaging, and user instructions
- Recordkeeping – Maintain technical files and DoC for post-market checks
Timeline for CE Marking
The timeline for CE Marking depends on the product category and whether a Notified Body is involved. For self-declared products, the process can be completed in 3 to 6 weeks. If external testing or assessments are needed, the process may take up to 12 weeks or more. Delays can occur if test reports are missing or technical documentation is incomplete. Regular file updates support faster audits and smoother customs processing.
What is the CE Marking Cost?
CE Marking costs vary based on product complexity, number of required tests, directive requirements, and whether third-party certification is needed. Simpler products with clear test results and complete documentation can be marked with lower expenses. However, products needing multiple lab tests, language translations, or Notified Body involvement may incur higher costs. These can include testing fees, technical writing, risk assessment consulting, and CE label adjustments.
How can Pacific Certifications Help?
Pacific Certifications assists manufacturers and distributors with the CE Marking process across various product categories. We work with your teams to identify applicable directives, evaluate design files, and compile the required technical documentation.
Our services include:
- Product category and directive assessment
- Risk analysis and conformity planning
- Guidance on harmonized standard selection
- Technical documentation review and validation
- Declaration of Conformity support
- Coordination with Notified Bodies when required
- Training for internal teams and post-market file maintenance
We are accredited by ABIS and trusted by companies across electronics, medical, machinery, and consumer goods segments for support in CE-related evaluations.
Training and Courses
Lead Auditor TrainingCovers internal auditing against CE-related directives, technical file verification, and market surveillance preparation.
Lead Implementer TrainingFocuses on developing internal systems for conformity assessment, documentation, and CE labeling management.
Internal Auditor TrainingIdeal for product design or QA teams to carry out periodic file reviews and supplier compliance checks.
Pacific Certifications provides accredited training programs. If your organization is looking for CE Marking training, our team is equipped to help you. Contact us at support@pacificcert.com
FAQs
Is CE Marking mandatory for all products in Europe?
No. Only products covered under specific CE directives require CE Marking. However, for those that fall under these directives, it is compulsory.
Can CE Marking be done without a Notified Body?
Yes, for many product types, CE is a self-declared process. However, certain products such as medical devices or PPE may need Notified Body involvement.
What happens if my product does not meet CE requirements?
It cannot legally be sold in the EU. Authorities can impose fines, ban imports, or request product recalls.
Is CE Mark accepted outside of Europe?
Yes, some countries outside the EU recognize CE Marking as a benchmark for safety, though it is not a substitute for local requirements.
How long is CE Marking valid?
There is no expiry, but updates to directives, changes in product design, or modifications in supply chain may require reassessment.
Contact Us
If you need support with CE Marking, contact us at support@pacificcert.com.
Read More at: Blogs by Pacific Certifications






