What is ISO/IEC 17025:2017?

ISO/IEC 17025:2017 is the international standard that outlines the general requirements for the competence, impartiality, and consistent operation of laboratories performing testing and calibration. It is widely recognized as the most critical accreditation for laboratories and forms the foundation of trust in their technical results. The standard applies to all organizations performing laboratory activities, regardless of the number of personnel or the extent of the scope of testing and calibration.
The objective of ISO/IEC 17025 is to promote confidence in laboratory operations and outcomes by ensuring they are technically valid and aligned with internationally accepted methods. It enables laboratories to produce accurate results and establish traceability, fostering both client and regulatory confidence.
For ISO/IEC 17025 certification audits tailored to your laboratory, contact our team at support@pacificcert.com
Purpose
The core purpose of ISO/IEC 17025 is to verify that laboratories are technically proficient and operate under a strong quality system. Unlike broader quality standards such as ISO 9001, ISO/IEC 17025 is specific to the testing and calibration sector. It helps laboratories consistently produce valid and reliable results and boosts international recognition through harmonized assessment protocols.
The standard is also designed to help laboratories improve efficiency, maintain impartiality in testing, and strengthen their ability to detect measurement errors or equipment malfunctions before they affect outcomes. Ultimately, it provides the backbone for reliable test results that underpin product quality, regulatory approvals and scientific accuracy.
Scope and Applicability
ISO/IEC 17025 applies to a wide variety of laboratories including those in industrial testing, environmental analysis, pharmaceutical validation, food safety, forensic science, and academic research. Whether operating in the private or public sector, any facility that performs testing, calibration or sampling as part of its services can benefit from implementing this standard.
The scope covers both management system requirements and technical requirements for labs, ensuring that quality assurance processes and technical competence are evaluated together. It also applies to both in-house laboratories and third-party service providers, especially where test results influence regulatory approvals, product release decisions or safety evaluations.
Key Definitions
- Calibration: Comparison of a measurement instrument to a known standard.
- Traceability: The ability to relate individual measurements to national or international standards.
- Impartiality: Freedom from conflict of interest or bias in laboratory operations.
- Measurement Uncertainty: Quantification of doubt in the result of a measurement.
- Validation: Confirmation that a method is appropriate for the intended use.
Clause-wise structure of ISO/IEC 17025:2017
Clause | Title | Description |
1 | Scope | Defines the applicability to testing, calibration and sampling labs. |
2 | Normative References | Lists relevant referenced documents. |
3 | Terms and Definitions | Contains definitions that apply within the context of this standard. |
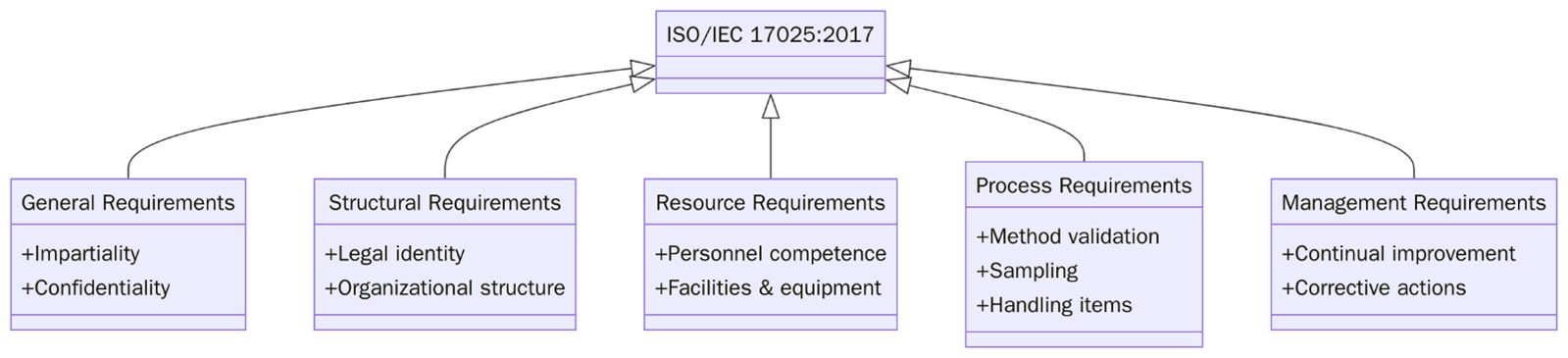
4 | General Requirements | Focuses on impartiality and confidentiality in lab operations. |
5 | Structural Requirements | Outlines the organizational and legal structure needed for lab activities. |
6 | Resource Requirements | Covers personnel qualifications, equipment, facilities and external services. |
7 | Process Requirements | Details testing/calibration method validation, result handling and reporting. |
8 | Management System Requirements | Focuses on quality assurance, document control and continual improvement. |
What are the requirements of ISO/IEC 17025:2017?
Before implementing ISO/IEC 17025, laboratories need to understand that the requirements are both technical and managerial. These requirements are meant to ensure that test outcomes are accurate and the management system supports quality and impartiality. Below are some of the key requirements:
- Establish and maintain impartiality by identifying and mitigating any conflicts of interest.
- Define roles and responsibilities across management and technical staff to support compliance.
- Ensure personnel competency through regular training and qualification verification.
- Validate test methods to confirm suitability for specific materials or conditions.
- Maintain environmental conditions that do not adversely impact test results.
- Calibrate equipment regularly and ensure measurement traceability to recognized standards.
- Document quality control procedures for reviewing data, addressing outliers and handling non-conforming work.
- Implement a management system that includes internal audits, customer feedback review and continual improvement mechanisms.
What are the benefits of ISO/IEC 17025:2017?
By following ISO/IEC 17025, labs show competence in their results while assuring customers and regulators that procedures meet international standards. These benefits help laboratories gain access to wider markets, reduce the risk of error and improve internal accountability. Below are some of the key benefits:
- Global recognition of testing reports and calibration certificates
- Increased client confidence in test validity and laboratory expertise
- Improved traceability and accuracy of test equipment and measurements
- More consistent testing outputs due to standard method validation
- Higher chances of regulatory acceptance and faster approval cycles
- Fewer customer complaints due to reliable testing procedures
- Strengthened internal audit processes to detect and prevent issues early
Across industries like pharmaceuticals, automotive, electronics, and food safety, ISO/IEC 17025 has become the benchmark for laboratory competence. Recent years have shown increased demand for accreditation due to global supply chain pressures and third-party validation needs.
With a growing focus on traceability and accountability in product testing, ISO/IEC 17025 is expected to see higher adoption, especially in biotech, environmental testing, and advanced materials sectors. The 2025 trend also points to more digital integration of lab data systems to support real-time verification and audit readiness
ISO/IEC 17025 Audit Checklist
The following checklist helps laboratories prepare for an audit aligned with ISO/IEC 17025:
- Are roles and responsibilities clearly defined for all staff?
- Is impartiality formally declared and reviewed?
- Are all equipment calibration records traceable to national or international standards?
- Have all test methods been validated and documented?
- Are procedures in place to handle complaints and non-conforming work?
- Are quality records securely maintained and regularly reviewed?
- Have internal audits been conducted within the required timeframes?
- Are training records current and competency assessments completed?
Certification Process and Procedure
To get certified, a testing or calibration lab generally goes through the following steps:
- Gap Analysis: Evaluate current operations against ISO/IEC 17025 requirements.
- System Development: Establish documentation and procedures to meet standard clauses.
- Implementation: Train staff, validate methods, and begin quality system practices.
- Internal Audit: Conduct internal audits and management reviews.
- Stage 1 Audit: Preliminary review by the certification body to assess readiness.
- Stage 2 Audit: Full assessment of system implementation, lab activities, and results.
- Certification Issuance: Upon successful audit, the certificate is issued and valid for 3 years.
- Surveillance Audits: Ongoing audits to ensure continued compliance.
To schedule an ISO/IEC 17025 audit, reach out to our team at support@pacificcert.com
ISO/IEC 17025 Certification Costs
The cost of ISO/IEC 17025 certification depends on the lab’s size, number of test methods, geographic location, and current management system maturity. Smaller labs may incur lower certification fees but require longer setup time if documentation is lacking. Organizations that already follow ISO 9001 or other standards may have reduced costs due to overlap in system requirements. External auditor fees, internal training time and periodic surveillance audits also affect the total cost.
Certification Timeline
Most labs can expect the certification process to take between 3 to 6 months depending on their preparedness. Labs with established procedures and partial ISO frameworks may accelerate implementation in as little as 2 months. The timeline includes the time taken for method validation, document creation, internal audits, and auditor scheduling. Surveillance audits typically occur annually, and full recertification is needed every 3 years.
How Pacific Certifications Can Help?
Pacific Certifications is accredited by ABIS and provides third-party certification audits for ISO/IEC 17025. We audit single-location labs, multi-site operations and testing providers across industries such as pharmaceuticals, chemicals, electronics, and industrial manufacturing.
Our audit team focuses on evaluating technical accuracy, process controls, and evidence of measurement traceability. We also assist in identifying areas where labs can better align their operations with the standard’s process and resource requirements.
To get ISO/IEC 17025 certified for your laboratory, contact us at support@pacificcert.com.
ISO/IEC 17025 Training and Courses
Various training courses are available to help laboratories meet ISO/IEC 17025 requirements, including:
- Lead Auditor Training – Equips professionals to conduct external third-party audits.
- Lead Implementer Training – For those responsible for planning and executing ISO/IEC 17025 implementation.
- Internal Auditor Training – Preparing internal auditors for certification audits
Pacific Certifications provides accredited training programs. If your laboratory is looking for ISO/IEC 17025 training, contact us at support@pacificcert.com
FAQs
Is ISO/IEC 17025 mandatory?
It is not legally mandatory, but required for many contractual and regulatory testing applications.
Can small laboratories get certified?
Yes, the standard is scalable and suitable for both small and large laboratories.
Does ISO/IEC 17025 cover sampling?
Yes, when it is part of the testing or calibration process.
How long is the certification valid?
It is valid for three years with annual surveillance audits.
Can ISO/IEC 17025 be integrated with ISO 9001?
Yes, both systems can operate together, especially in labs already certified to ISO 9001.
Contact Us
If you need support with ISO/IEC 17025:2017 certification, contact us at support@pacificcert.com.
Read More at: Blogs by Pacific Certifications






