What is ISO 14971:2019?

ISO 14971:2019 is the international standard that outlines a comprehensive framework for the risk management of medical devices throughout their lifecycle. It provides manufacturers with a systematic approach to identifying hazards, estimating and evaluating risks, controlling those risks, and monitoring the effectiveness of those controls.
This standard is essential for meeting regulatory requirements in global markets, including those set by the U.S. FDA, European MDR/IVDR, Health Canada, TGA, and others. ISO 14971 emphasizes that risk management is an integral part of the design, development, production, post-market surveillance, and even decommissioning of medical devices.
ISO 14971 is specifically focused on patient safety and compliance, ensuring that devices are both effective and safe for use in real-world conditions.
Looking to implement ISO 14971 for your medical device manufacturing process? Contact us at support@pacificcert.com!
Scope and Applicability
ISO 14971 is applicable to all types of medical devices, including:
- Active and non-active medical devices
- In vitro diagnostic (IVD) devices
- Software as a Medical Device (SaMD)
- Combination products (e.g., drug-device products)
- Implantable devices and Class III risk category products
It applies to the entire product lifecycle, from initial design and development to manufacturing, clinical use, and post-market activities such as complaint handling and field safety corrective actions. If your organization designs, develops, manufactures, or distributes medical devices, ISO 14971 is critical for both compliance and patient safety.
Need to assess whether your device falls under ISO 14971 requirements? Contact us at support@pacificcert.com.
Certification Process
ISO 14971 compliance is mandatory for regulatory approvals and is often verified during ISO 13485 certification audits.
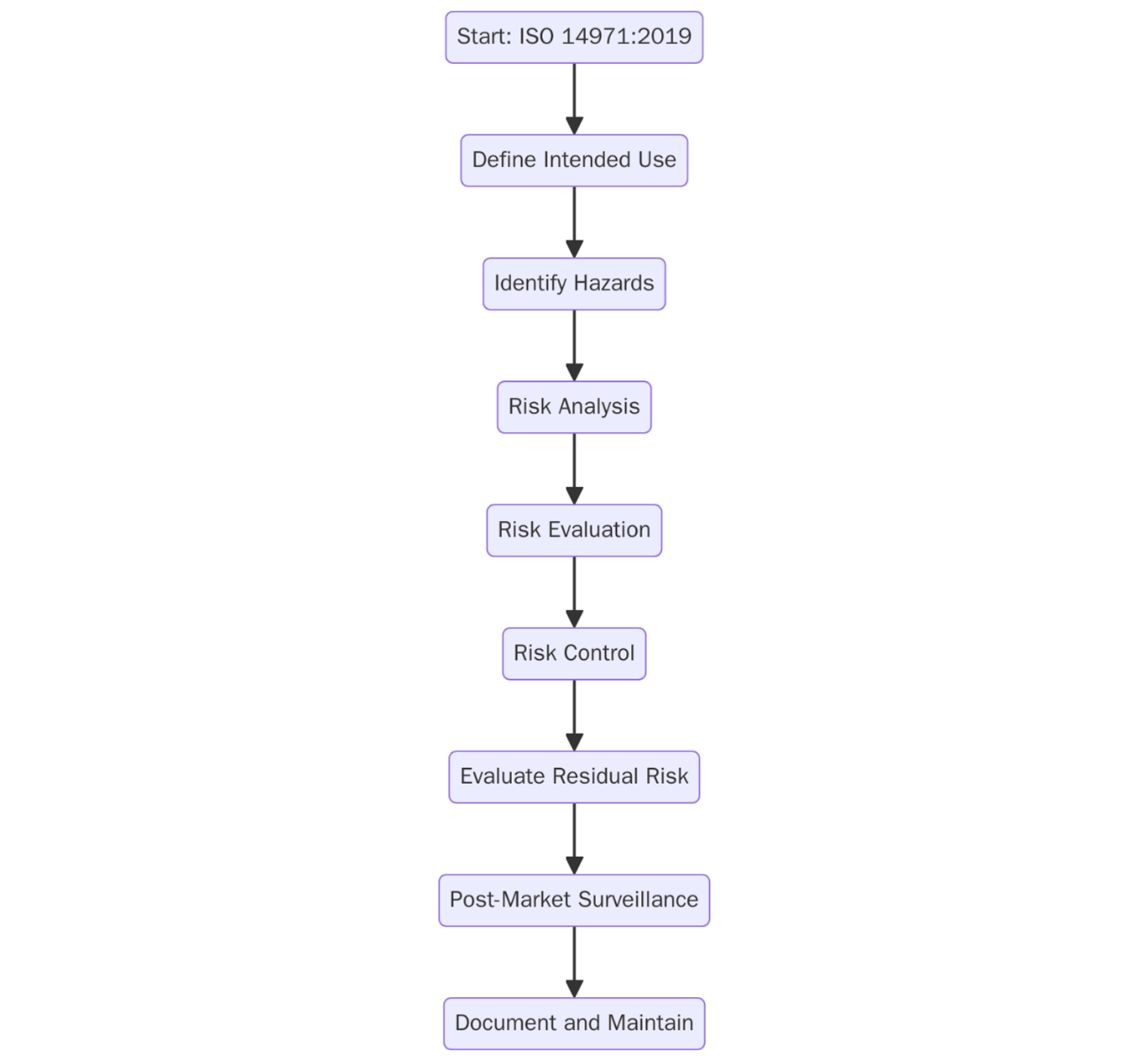
Typical steps toward compliance include:
- Risk Management Planning – Establish a risk management policy and define criteria for risk acceptability.
- Hazard Identification – Systematically identify known and foreseeable hazards associated with the device.
- Risk Analysis – Estimate the risks for each hazard, considering both probability and severity.
- Risk Evaluation – Determine whether risks are acceptable based on predefined criteria.
- Risk Control – Identify and implement measures to reduce risk, then verify their effectiveness.
- Residual Risk Evaluation – Assess whether remaining risks are acceptable.
- Risk Management Report – Summarize all activities, decisions, and residual risk justifications.
- Post-Market Monitoring – Collect and analyze feedback, incidents, and field data to update the risk profile.
We at Pacific Certifications assist manufacturers in aligning their quality and risk systems with ISO 14971, supporting regulatory submissions and audits. Contact us at support@pacificcert.com.
How to Implement ISO 14971:2019 in Your Organization?
Implementation involves integrating risk management into product development, design controls, and quality management processes. Begin by forming a cross-functional risk management team with expertise in engineering, clinical application, quality assurance, and regulatory affairs.
Use tools such as FMEA (Failure Mode and Effects Analysis), FTA (Fault Tree Analysis), and Hazard Analysis to assess device-related risks. Document your entire risk management process, from planning through to post-market feedback. Align your process with regulatory expectations such as Annex I of the EU MDR and FDA’s CFR Part 820.
Ensure that design inputs, verification, validation, and clinical data address identified risks. Finally, use the risk management file (RMF) as a living document throughout the device lifecycle.
Need support integrating ISO 14971 with ISO 13485 and global regulations? Contact us today support@pacificcert.com!
Documentation Required
Compliance with ISO 14971:2019 requires a detailed Risk Management File (RMF), which includes:
- Risk management plan
- List of identified hazards
- Risk analysis and evaluation records
- Risk control measures and verification data
- Residual risk assessments
- Benefit-risk analysis (if residual risks are significant)
- Risk management report
- Post-market surveillance and feedback logs
We offer certification support for ISO 14971, ISO 13485 and other management system certifications, contact us at support@pacificcert.com.
Eligibility Criteria for ISO 14971:2019
ISO 14971 is applicable to:
- Original Equipment Manufacturers (OEMs)
- Contract design and manufacturing organizations (CDMOs)
- Software developers of medical applications (SaMD)
- Distributors and importers involved in complaint handling
- Startups preparing for CE marking, FDA clearance, or international market entry
Not sure if your device or process must meet ISO 14971? Get in touch with us at support@pacificcert.com!
ISO 14971:2019 Certification Costs
While ISO 14971 is necessary for ISO 13485 certification and market approvals. The cost of implementation depends on:
- Device classification (I, IIa, IIb, III)
- Size and complexity of the product portfolio
- Depth of existing quality and risk systems
- Integration with ISO 13485 or other QMS standards
- Post-market data and field support capabilities
Contact us at support@pacificcert.com for a customized cost estimate!
Certification Timeline (for Integration with ISO 13485)
Week | Activities |
Week 1 | Risk management planning and team formation |
Week 2 | Hazard identification and preliminary risk analysis |
Week 3 | Risk evaluation and risk control implementation |
Week 4 | Documentation review and risk management report preparation |
Week 5 | Internal audit and integration with ISO 13485 QMS |
Week 6 | Third-party ISO 13485 audit and regulatory submission support |
We help streamline your ISO 14971 implementation and align it with market entry timelines. Contact us at support@pacificcert.com!
Clauses of ISO 14971:2019
Clause 1 – Scope
Defines the application of the standard to all stages of the device lifecycle and across all device types. It reinforces risk-based thinking as a foundational principle.
Clause 2 – Normative References
Specifies ISO 14971 as a standalone standard with no other referenced standards required for compliance.
Clause 3 – Terms and Definitions
Clarifies essential terminology, including “hazard,” “harm,” “residual risk,” “benefit-risk,” and “risk control,” which must be uniformly understood by all team members.
Clause 4 – General Requirements for Risk Management
Requires organizations to establish a documented risk management process, assign responsibilities, and ensure top management oversight throughout the lifecycle.
Clause 5 – Risk Analysis
Outlines steps to identify hazards, estimate risks, and document cause-effect scenarios using scientific, clinical, and statistical data.
Clause 6 – Risk Evaluation
Instructs manufacturers to compare estimated risks with defined acceptability criteria and make decisions about further action.
Clause 7 – Risk Control
Focuses on selecting and implementing controls to reduce risks and verify their effectiveness. Priority must be given to inherently safe design, followed by protective measures, and finally user training.
Clause 8 – Evaluation of Overall Residual Risk
Guides the evaluation of cumulative residual risks and, if applicable, the need for benefit-risk justification.
Clause 9 – Risk Management Review
Mandates a final review to confirm all planned activities are complete and residual risks are acceptable before commercial release.
Clause 10 – Production and Post-Production Activities
Requires ongoing post-market monitoring, complaint handling, vigilance reporting, and real-world data analysis to update the risk profile continuously.
Need help applying these clauses to your product lifecycle? Contact us at support@pacificcert.com.
ISO 14971:2019 Requirements
- Establish and maintain a risk management process throughout the product lifecycle
- Define risk acceptability criteria based on intended use and regulatory obligations
- Identify all known and foreseeable hazards during design and development
- Evaluate risk probability and severity, both before and after controls
- Implement verified risk control measures (design changes, warnings, training)
- Document residual risk and benefit-risk decisions
- Continuously monitor and update the risk profile using post-market data
- Maintain a comprehensive risk management file
- Integrate risk management with ISO 13485:2016 quality management systems
We help align your processes with these requirements, reach us today at support@pacificcert.com.
Benefits of ISO 14971:2019
- Supports CE marking, FDA submissions, and compliance with global MDRs and IVDRs.
- Systematic hazard identification and mitigation reduce the risk of harm and product recalls.
- Integrating risk management early accelerates development and approval processes.
- Risk-informed design leads to more robust, effective medical devices.
- Ongoing data-driven risk evaluation enables quicker responses to field issues.
- Meeting ISO 14971 helps gain access to global markets with varying regulatory expectations.
- Promotes system-wide consistency, efficiency, and audit readiness.
- Transparent risk management enhances confidence among clinicians, regulators, and users.
This year, medical device regulators are increasing their scrutiny of risk-benefit analysis and real-world performance. ISO 14971 is now directly referenced in the EU MDR (Annex I), FDA guidance documents, and IMDRF frameworks. According to a recent study by MedTech Europe, over 85% of nonconformities in device audits involved inadequate risk documentation or post-market follow-up.
There is also growing emphasis on software-based devices (SaMD) and AI-enabled diagnostics, where risk profiles evolve rapidly and ISO 14971 is being adapted to support agile development and real-time monitoring.
To maintain market access, mitigate product liability, and ensure ethical clinical use, ISO 14971 compliance is now more critical than ever.
Want to future-proof your risk management systems? Contact us at support@pacificcert.com!
How Pacific Certifications Can Help?
We offer full support for ISO 14971 integration and compliance:
- Risk management framework development
- Cross-functional training (engineering, QA, RA, clinical)
- Documentation templates and risk file creation
- Internal audits and gap assessments
- Alignment with ISO 13485, EU MDR, FDA QSR
- Post-market surveillance integration
From startups to global manufacturers, we help you manage risk confidently and compliantly. Start your ISO 14971 project today. Contact us at support@pacificcert.com.
FAQ on ISO 14971:2019
Is ISO 14971 certifiable?
ISO 14971 is issued in compliance certifications and is essential for ISO 13485 certification and regulatory compliance.
Is it mandatory under EU MDR or FDA?
Yes, ISO 14971 is recognized and often required as evidence of risk management compliance. Yes, ISO 14971 is recognized and often required as evidence of risk management compliance.
Can it be applied to software-only devices?
Absolutely. It is fully applicable to Software as a Medical Device (SaMD).
How does it relate to ISO 13485?
ISO 14971 supports and integrates with ISO 13485’s risk-based approach.
What tools are used for compliance?
FMEA, FTA, Hazard Analysis, and Root Cause Analysis are commonly used techniques.
Ready to get ISO 14971 certified?
Contact Pacific Certifications to begin your certification journey today!
Suggested Certifications –
Read more: Pacific Blogs






